Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Treating Nuclear Waste With Sulfides
March 10, 2008
| A version of this story appeared in
Volume 86, Issue 10

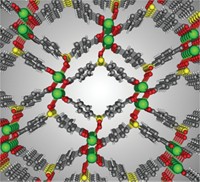
Metal sulfides may make it easier to remove radioactive strontium-90 from nuclear waste (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0711528105). To minimize the need for waste storage space, handlers would like to be able to remove 90Sr from bulk waste so they can immobilize and store just the radioactive component. Common adsorbents, however, may decompose in the extremely alkaline environment of many waste streams. These streams also have high salt concentrations that challenge selective uptake of Sr2+. Now, a research group led by Mercouri G. Kanatzidis of both Northwestern University and Argonne National Laboratory has found that layered metal sulfides may circumvent those problems. The material, K2xMnxSn3-xS6 (x = 0.5-0.95, shown), is composed of MnS6 and SnS6 octahedra connected through the sulfur ligands with exchangeable K+ ions located between the layers. The researchers found that the layered sulfide removed more than 92% of Sr2+ from solutions ranging from pH 3.2 to pH 14.0. The material was also selective for Sr2+ in strongly alkaline environments incorporating cations from Groups 1 and 2.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter