Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Aromatic Chloride Catcher
March 10, 2008
| A version of this story appeared in
Volume 86, Issue 10
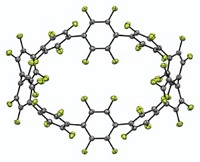
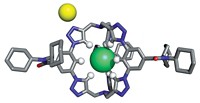
To grab hold of an anion, an organic host molecule usually uses strong hydrogen-bond donors, such as N–H bonds or C–H bonds adjacent to a cationic center. Now, chemists at Indiana University have shown that a neutral macrocycle equipped with only aromatic C–H bonds (which are weak H–bond donors) can snatch up a chloride anion with an affinity akin to its stronger H–bonding brethren (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200704717). Using "click chemistry" and other reactions, Amar H. Flood and Yongjun Li created a neutral macrocycle wherein four phenyl rings alternate with four triazoles. Each of these eight rings donates one C–H bond for catching the chloride ion (complex shown). Flood and Li attribute the macrocycle's surprisingly good affinity for chloride ions to the triazole moieties' strong dipoles and to a central cavity that's the perfect size for the anion. The click chemistry-based synthesis makes it easy to create libraries of such macrocycles, which could be used to sequester other anions, Flood says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter