Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
A Catalytic First
Bacteria master reaction by making the substrate a radical
by Sarah Everts
March 17, 2008
| A version of this story appeared in
Volume 86, Issue 11
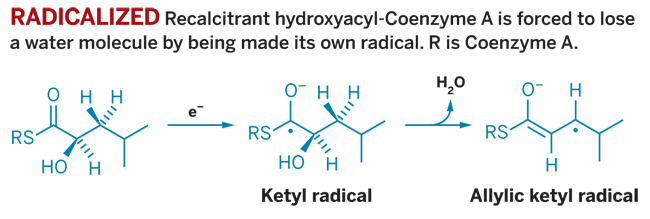
A COMMON ANAEROBIC MICROBE in the human gut has been caught doing some curious biochemistry that had been previously postulated but never proven. Clostridium difficile has an enzyme that pulls off a difficult reaction needed for energy metabolism by turning its substrate directly into a radical, a new report shows (Nature, DOI: 10.38/nature06637).
Typically, substrate radicals involved in enzyme-catalyzed reactions are generated through radical organic cofactors or active-site amino acids. C. difficile provides the first case in which an enzyme radicalizes its substrate directly via simple electron transfer, comments Joseph T. Jarrett, a chemist at the University of Hawaii, Manoa, in an associated commentary in Nature.
The team of researchers, led by Antonio J. Pierik, a biochemist at Philipps University in Marburg, Germany, also shows that the substrate radical is produced through a reduction reaction rather than an oxidation, contrary to standard textbook redox activation. "Most enzymes that are known to generate radicals do so by removing a hydrogen atom from the substrate," Jarrett notes.
The unusual C. difficile reaction is undertaken by an enzyme called 2-hydroxyisocaproyl-CoA dehydratase, which converts leucine into short-chain fatty acids.
Metabolizing leucine in the absence of oxygen is difficult, partially because midway through the process a stalwart 2-hydroxyacyl-CoA intermediate is created. This 2-hydroxyacyl-CoA cannot be reduced further until it can shake off a water molecule. That's when the dehydratase steps in.
Using an electron from the iron-sulfur clusters in its active site, the enzyme turns the 2-hydroxyacyl-CoA into a ketyl radical that can shed the crucial water. The radical product is oxidized to give an isocaprenoyl derivative that can then be further reduced.
"Time will tell whether this newly discovered radical mechanism is used by other enzymes," Jarrett notes. "But the authors have shown that even in the relatively mature field of enzymology, there is still much to learn."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter