Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Alternative Route To Dehydroalanine
March 31, 2008
| A version of this story appeared in
Volume 86, Issue 13
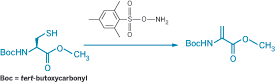
Dehydroalanine (Dha), which can be formed by oxidative elimination of alkylated derivatives of cysteine (Cys) or selenocysteine, provides a chemical handle for selectively modifying proteins and thereby studying the process of posttranslational modifications. The usual strategies for forming Dha rely on peroxide-induced oxidative elimination, which can unintentionally affect methionine as well. Benjamin G. Davis and coworkers at the University of Oxford instead use O-mesitylenesulfonylhydroxylamine (MSH) for the oxidative elimination reaction (J. Am. Chem. Soc., DOI: 10.1021/ja800800p). When the researchers treated Boc-protected Cys methyl ester with excess MSH (shown), the Dha derivative formed at almost quantitative yield in just a few minutes at room temperature in open air. They used the strategy to convert the single exposed Cys on the surface of subtilisin from the bacterium Bacillus lentus without oxidizing any of the three methionine units also present. The researchers then tethered various posttranslational modifications to the protein by adding thiols to the Dha. In one case, they regenerated Dha from the resulting thioether by a similar oxidative elimination using MSH, allowing a "functional switch" on the protein surface.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter