Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Size-Selective Acid Catalysis
Framework pores control substrate access to exposed metal ions
by Stephen K. Ritter
April 21, 2008
| A version of this story appeared in
Volume 86, Issue 16
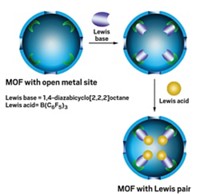
Metal-organic frameworks (MOFs), once synonymous only with hydrogen gas storage, are starting to show their potential as selective catalysts. In the latest development on this front, Jeffrey R. Long and coworkers at the University of California, Berkeley, have demonstrated how to take advantage of a MOF's pore size to precisely select substrates for heterogeneous catalytic reactions (J. Am. Chem. Soc., DOI: 10.1021/ja800669j).
MOFs are porous crystalline materials typically made up of multimetal clusters interconnected by organic linkers. The researchers began their study by probing the catalytic activity of a manganese benzenetristetrazolate MOF (Mn-BTT), a material Long's group first synthesized in 2006. This MOF started out as a record-setting H2 storage material, with part of its success owing to two different types of unsaturated Mn2+ ions left exposed within the pore structure, Long says.
Besides being good at snagging H2 molecules, the Mn2+ ions are Lewis acids and are well positioned to interact with guest molecules that are small enough to enter the framework pores, he adds. The team reasoned that Mn-BTT could function as a stand-in for zeolites, an industrially relevant class of microporous heterogeneous catalysts.
To prove the idea, Long and research group members Satoshi Horike, Mircea Dinc , and Kentaro Tamaki carried out two sets of reactions that rely on Lewis acid catalysts. One reaction was the cyanosilylation of aromatic aldehydes or ketones to form cyanohydrins, while the other was the Mukaiyama aldol reaction that couples aldehydes with silyl enol ethers to form β-hydroxyketones.
, and Kentaro Tamaki carried out two sets of reactions that rely on Lewis acid catalysts. One reaction was the cyanosilylation of aromatic aldehydes or ketones to form cyanohydrins, while the other was the Mukaiyama aldol reaction that couples aldehydes with silyl enol ethers to form β-hydroxyketones.
The MOFs turned out to function just as the researchers imagined they would. For example, cyanosilylation of an aryl aldehyde containing a small aryl group, such as a phenyl, went essentially to completion—a significant improvement in yield over other MOF catalysts. On the other hand, when the larger biphenyl group was used with an aryl methyl ketone substrate, the yield dropped to nearly zero, presumably because the substrate was too large to enter the MOF's pores and come in contact with the metal sites.
"Developing new catalysts is one of the most important challenges in the chemical industry, and the studies carried out by Long and coworkers illustrate well the power of being able to tailor active sites within MOFs," comments UCLA's Omar M. Yaghi, a leading authority on MOFs. "Their findings are along the lines of what researchers have envisioned to be possible by designing MOFs."
Using MOFs for catalysis has enormous potential, Long says. Compared with zeolites, their surface area is much higher, leading to greater catalytic efficiency on a weight basis. And simple modification of the bridging ligands and the ability to readily exchange the metal ions provide a tunability of properties that is lacking in zeolite chemistry, he says.
Zeolites are mostly made from silicon, aluminum, and oxygen—in essence they are nanoporous rocks and are very robust, explains Northwestern University's Joseph T. Hupp, another MOF researcher. That's why they are used as catalysts in many high-temperature, gas-phase refinery processes, he says.
Where MOFs really shine is in permitting chemists to retain the microporous character of zeolites while creating more versatile materials for condensed-phase catalysis, Hupp adds. And that makes MOFs promising for high-value transformations under milder conditions, such as enantioselective reactions for synthesizing pharmaceutical intermediates and other fine chemicals, he says.
"Given the broad utility of zeolites in catalysis, I believe the possible applications for MOFs in this area have really only just begun to be explored," Long says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter