Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Asymmetric Route To α-Amino Acid Esters
May 19, 2008
| A version of this story appeared in
Volume 86, Issue 20
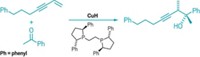
Developed in 1997 by Nicos A. Petasis of the University of Southern California, the Petasis reaction (shown; R substituents are various organic groups) is a multicomponent condensation of boronic acids with amines and aldehydes. Although that reaction is not catalytic, it has provided a way to make α-amino acids, and medicinal chemists often have relied on it. Now, Sha Lou and Scott E. Schaus of Boston University's Center for Chemical Methodology & Library Development report developing the first asymmetric catalytic version of the Petasis reaction. It uses chiral biphenol catalysts to convert alkenyl boronates, secondary amines, and glyoxylates to chiral α-amino acid esters with good yields and high enantiomeric ratios (J. Am. Chem. Soc., DOI: 10.1021/ja8018934). The advance eliminates the need for stoichiometric chiral starting materials and opens the way to a broader range of products.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter