Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Tailor-Made Crystals
Structure-directing synthesis increases fraction of reactive facets
by Mitch Jacoby
June 2, 2008
| A version of this story appeared in
Volume 86, Issue 22

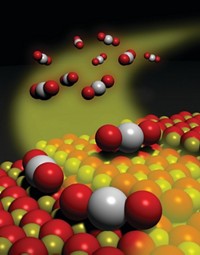
SCIENTISTS IN AUSTRALIA have developed a method that allows them to engineer crystals with an unusually large fraction of reactive facets. The research team demonstrated that crystals of the anatase form of titanium dioxide can be manipulated in a way that decreases the population of nonreactive facets that tend to dominate the surface and increases the population of reactive facets that ordinarily are scarce (Nature 2008, 453, 638).
Crystals with structures and other properties that are custom designed can serve as model systems to probe surface phenomena, including reaction mechanisms. Exploring these tailor-made solids and the methods to prepare them could also lead to advances in applications such as catalysis and photovoltaics.
Not only do the various facets of a crystal look similar to the naked eye, they also resemble one another at the microscopic level. In terms of composition, for example, no matter how a diamond or other crystal is cut, all of its faces are made of the same elements. Yet despite those similarities, subtle differences in the geometric arrangement of the atoms in one crystal face relative to another can lead to large differences in the chemical, electronic, and other properties of those two faces.
Surface scientists have known for years that those differences render certain faces of a given crystal catalytically active while other faces of the same crystal are largely inactive. The so-called (101) crystal faces of anatase TiO2, for example, are thermodynamically stable, highly abundant (typically around 95% of the crystal surface), and largely nonreactive. In contrast, the less stable minority (001) faces readily catalyze a water-splitting reaction.
In the past, researchers tried various ways to direct crystal growth to favor specific facets. Those efforts have been only partially successful. Now, Huagui Yang, Chenghua Sun, and Gaoqing (Max) Lu of the Autralian Institute of Bioengineering & Nanotechnology at the University of Queensland and coworkers have synthesized high-purity anatase TiO2 crystals with nearly 50% (001) facets.
On the basis of theoretical calculations that evaluated the effects of 12 types of adsorbates on surface energy and crystal growth, the team determined that the presence of fluorine on the crystal faces would increase the fraction of (001) facets. Accordingly, the group developed a synthesis based on TiF4 and hydrofluoric acid that yields the (001)-facet-enriched crystals. They also devised a heat treatment that subsequently drives off fluorine and leaves pristine TiO2 crystals.
The study is "convincing and compelling, with solid experimental evidence and theoretical underpinnings," says Scott A. Chambers of Pacific Northwest National Laboratory. He adds that if the new synthesis can be applied widely, then applications in catalysis and other areas "are very real possibilities."
Tulane University physics professor Ulrike Diebold remarks that the study provides an important step between experiments on nanomaterials, which are used in photocatalysis and dye-sensitized solar cells, and surface science experiments on macroscopic crystals. "I can imagine that this concept will find wide use, not only for TiO2, but for other metal oxides as well," she says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter