Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Three Protein Copper Sites Interconnected
June 9, 2008
| A version of this story appeared in
Volume 86, Issue 23

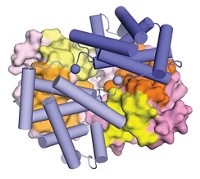
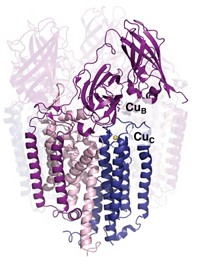
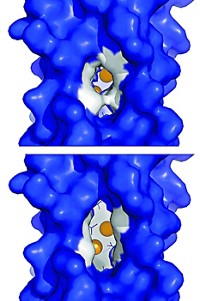
The copper sites in cupredoxins, a family of proteins that facilitate a variety of redox activities, are structurally and functionally diverse. As little as 10% of their amino acid sequence is identical. Nevertheless, the copper sites are always found within a similar β-barrel protein structure and may be evolutionarily linked. Studies on the binuclear CuA (purple) site of nitrous oxide reductase (N2OR) by Masha G. Savelieff, Yi Lu, and coworkers at the University of Illinois, Urbana-Champaign, now provide experimental evidence for that connection (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0711316105). The researchers added copper to metal-free protein and observed by electronic absorption and electron paramagnetic resonance spectroscopy that copper was first incorporated almost equally in separate type 1 (blue) and type 2 (red) sites. Over time the separate sites converted to the binuclear CuA structure. “These observations suggest that the purple CuA site contains the essential elements of type 1 and type 2 copper centers” and provide insight into the evolutionary relationships among cupredoxin proteins, the authors write.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter