Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Route To α-Alkylated Ketones
June 16, 2008
| A version of this story appeared in
Volume 86, Issue 24
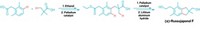
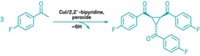
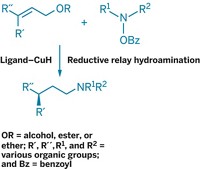
A new method for asymmetrically α-alkylating ketones—adding alkyl substituents to carbons adjacent to ketone groups to give chiral products—is simpler and more amenable to scale-up than current approaches, according to Duke University's Daniel Lim and Don M. Coltart, the researchers who devised the procedure (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200800848). In the reaction, a ketone is combined with a chiral N-amino cyclic carbamate (ACC, red) to generate a hydrazone, which is then alkylated by an alkyl halide (purple) to yield chiral products and the original ACC. This approach also makes it possible for the first time to α,α-bisalkylate ketones asymmetrically. The technique could prove useful for modifying and synthesizing alkylated ketone structures found in many natural products and drugs. The method works at temperatures between −40 and 0 °C, and the ACC chiral auxiliaries used can be directly recycled to save costs. Both of these factors make scale-up more practical. In previous asymmetric ketone α-alkylations, colder reaction temperatures (−110 to −78 °C) were required and the chiral auxiliaries used were more difficult to recycle.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter