Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Dalton Crosses The Pond
European inorganic chemistry meeting comes to Berkeley to showcase main-group chemistry
by Elizabeth K. Wilson
July 28, 2008
| A version of this story appeared in
Volume 86, Issue 30
THE DALTON DISCUSSIONS, venerable, intimate yearly conferences focusing on inorganic chemistry and sponsored by the Royal Society of Chemistry (RSC), have always been held in Europe.

Not this year. The 11th Dalton Discussions (DD11) for the first time ventured across the pond, all the way to the University of California, Berkeley, to highlight the renewed and invigorated field of main-group chemistry.
Main-group chemistry, that broad subfield of inorganic chemistry that includes groups 1, 2, and 13 to 18 on the periodic table, was once considered rather dry. Traditionally, when people heard of main-group chemistry, "they thought of very old chemistry," says John Arnold, a chemistry professor at UC Berkeley. What would come to mind are staid compounds that weren't considered to be very reactive, such as cements and silicates.
But that's no longer the picture. Main-group chemistry is experiencing an ongoing renaissance as chemists devise syntheses of ever more elaborate molecules with atoms such as boron, fluorine, mercury, and tin that have exotic bonding characteristics and intricate structures.
"The message of this meeting is there is lot of very interesting new chemistry associated with main-group elements," says Arnold, a coorganizer of the conference.
UC Berkeley, the UC system's flagship campus, seemed a natural locale choice for the meeting organizers because of the university's "large community of synthetic chemists who work in the area of main-group chemistry," says Rebecca Quine, manager of conference promotions and logistics for RSC.
DD11 built upon the foundation laid by a large main-group chemistry symposium held at the 2004 American Chemical Society meeting in Anaheim, Calif., in honor of the 70th birthday of University of Texas, Austin, chemistry professor and modern main-group chemistry pioneer Alan H. Cowley (C&EN, May 10, 2004, page 39). Since then, the field has continued to burgeon, particularly in areas such as materials chemistry, hydrogen storage, polymers, and nanowires, Arnold says.
The unusual format of the Dalton Discussions allows intense interactions that are not always possible in a typical meeting, participants say. The format is modeled after that of another RSC meeting, the Faraday Discussions, which focus on physical chemistry.
Rather than a series of moderate-sized talks, each Dalton Discussions session leads off with a keynote speaker, followed by three or four very short presentations. To bring participants up to speed on each others' work even before the meeting convenes, all of the talks are prepublished in a special issue of Dalton Transactions.
After the presentations come conversations. Attendees sit together, and the discussion centers around chemistry as a whole, rather than just one talk, Arnold says.
"It's like being back in graduate school and having a group meeting," says Frances H. Stephens, a chemist at Los Alamos National Laboratory who presented a poster at the meeting. "It is very interactive, and you have a lot of time to talk to people about the intricacies of their research."

HYDROGEN STORAGE materials (C&EN, Jan. 28, page 67) generated a lot of buzz at the meeting, as did silicon nanowires (C&EN, Jan. 14, page 12) and polymers (C&EN, Aug. 6, 2007, page 9).
From the German labs of University of Regensburg chemistry professor Manfred Scheer (C&EN, Oct. 22, 2007, page 48) and University of Karlsruhe chemistry professors Hansgeorg Schnoeckel and Andreas Schnepf came reports of new metalloid clusters. "The complexity of these molecules is really unprecedented," Arnold says.
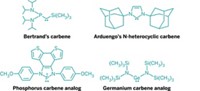
Boron, which is similar to carbon yet electron deficient, continues to play a prominent role in main-group chemistry. For example, Frieder Jäkle, chemistry professor at Rutgers University, described compounds that contain two boron atoms that form a rigid bridge between two ferrocene groups. His team varied the properties of these complexes by changing constituents on the bridge. By placing electron-withdrawing groups on the boron, for instance, Jäkle reported that he could increase interactions between the ferrocene moieties.
The molecules have numerous potential uses, Jäkle says. Ferrocenes, in addition to being very rigid—which allows them to preserve the complex's geometry—can be reversibly oxidized. Oxidizing and reducing the ferrocene groups changes the shape of the molecules, making them potentially useful as molecular machines or actuators. And boron's ability to bind substrates could make the compounds potentially useful for Lewis acid catalysis and for sensing anions such as fluoride, an anticavity additive in drinking water and toothpaste that could be toxic in large quantities.
A GROUP led by François P. Gabbaï, a chemistry professor at Texas A&M University who organized the meeting's poster session with sponsorship from the ACS Division of Inorganic Chemistry, is designing boron-based receptors that bind with fluorine ions that might also be eventually developed for use in fluoride sensors.
In one of his research projects, Gabbaï is working on chelators that rely on two binding sites to grab a target atom. One of Gabbaï's strategies employs electron-deficient boron as one binding site and mercury as the other. Despite mercury's potential toxicity, Gabbaï says, its complexes are stable and compatible with water, and their penchant for phosphorescing at room temperature makes them good candidates for fluoride sensors.
RSC has DD12 in the works, and the organization says it is looking at possibly holding future meetings, including Faraday Discussions, in the U.S., Quine says. "The discussion format, especially, is something that American audiences warm to," she observes.
- Dalton Crosses The Pond
- European inorganic chemistry meeting comes to Berkeley to showcase main-group chemistry
- ACS Inorganic Division Adds U.S. Touch To Dalton Discussions
- The Royal Society of Chemistry's traditionally Europe-based inorganic chemistry meeting, Dalton Discussions, had a nontraditional U.S. locale this year



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter