Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Water Whips Proteins Into Shape
August 18, 2008
| A version of this story appeared in
Volume 86, Issue 33
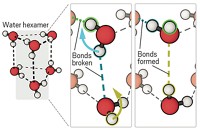
With the aid of far-infrared spectroscopy, researchers have taken a detailed look at how water molecules choreograph the intricate process of protein folding (Angew. Chem. Int. Ed. 2008, 47, 6486). A team led by Martin Gruebele at the University of Illinois, Urbana-Champaign, and Martina Havenith at Ruhr University, in Bochum, Germany, adapted a laser-based far-IR technique to monitor solvent dynamics during protein folding in real time. The researchers focused ultrashort laser pulses onto a solution of unfolded ubiquitin, a common regulatory protein, and initiated folding by diluting a denaturant in the buffer. The spectroscopic technique, which the team calls kinetic terahertz absorption, provided a picosecond-by-picosecond glimpse of water-protein hydrogen bonds breaking, as well as their replacement by protein-protein hydrogen bonds. This rearrangement helps organize the structure of a folding protein. The team found that motion from the solvent network of water molecules helped set up the right configuration, facilitating ubiquitin’s folding. The experimental data corroborates other researchers’ hypotheses that some protein and solvent dynamics are intertwined, the authors write.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter