Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Copper Uncaged
September 1, 2008
| A version of this story appeared in
Volume 86, Issue 35
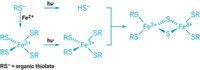
Caged-copper complexes that release their metal cargo on cue may help scientists explore how this essential yet toxic transition metal is trafficked and utilized in cells (J. Am. Chem. Soc., DOI: 10.1021/ja8047442). Katherine J. Franz and coworkers at Duke University envision that their caged-copper strategy also could be harnessed to kill cancer cells. That's because the method could permit precise site- and time-dependent delivery of the metal, which promotes the formation of toxic reactive oxygen species that can lead to cell death. To make the caged complexes, the researchers embedded a photoactive nitrophenyl group into the backbone of a nitrogen-rich tetradentate copper-chelating compound. Upon exposure to ultraviolet light, the chelator's backbone breaks in two, uncaging the copper (shown). Although the chelator grips copper with picomolar affinity, that might not be tight enough to keep biological copper-binding proteins from making off with the copper. So Franz's team is now working to improve the stability of caged-copper complexes and to ensure that they can be delivered to their desired cellular destinations.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter