Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Subnanometer Gold Cluster Catalysts
September 8, 2008
| A version of this story appeared in
Volume 86, Issue 36
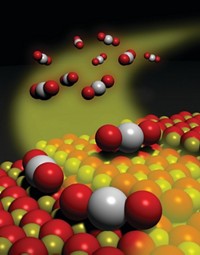
Tiny gold clusters—just two atomic layers thick and roughly 0.5 nm in diameter—are far more active room-temperature CO oxidation catalysts than microscopic gold clusters of many other sizes and thicknesses, according to a study (Science 2008, 321, 1331). The investigation provides new details about the high catalytic activity exhibited by nanoparticles of gold, a metal that in bulk form is inert. An international research team led by Christopher J. Kiely of Lehigh University and Graham J. Hutchings of Cardiff University, in Wales, used traditional chemical methods to disperse gold particles on iron oxide supports and subjected the samples to various heat treatments. The procedures yielded a series of samples with widely varying catalytic activities and particle sizes ranging from individual atoms to one- and two-layer clusters several nanometers in diameter. On the basis of state-of-the-art microscopy and other analytical methods, the group concludes that the high catalytic activity can be attributed primarily to 0.5-nm bilayer clusters containing approximately seven to 10 gold atoms.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter