Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Bottling Hydrogen In Solids
Basic research yields better hydrogen-storage materials, but the best is yet to come
by Mitch Jacoby
January 28, 2008
| A version of this story appeared in
Volume 86, Issue 4
WHAT A CONVENIENCE it would be to travel like Harry Potter. In the popular book and movie series, the boy-wizard and other characters in his magical world suddenly vanish from one location and instantly arrive elsewhere, apparently without the slightest expenditure of effort—not to mention fuel.
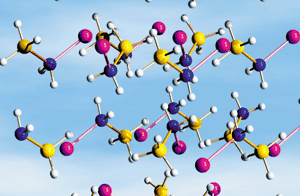
Hydrogen-evolving solids such as lithium amidoborane (LiNH2BH3) may serve as fuel-storage media.
For the rest of us, traveling by more ordinary means—for example in automobiles—usually requires filling our tanks with gasoline. But given today's near-record-high oil prices, a fill-up at the gas pump can cost a small fortune. That's just one of the reasons that researchers in many countries have been working on technologies to replace fossil fuels with alternatives, including element number one on the periodic table—hydrogen.
Another motivation to bring hydrogen into the fuel fold is reducing greenhouse gas emissions and other harmful environmental effects. And in the U.S., where nearly 60% of the petroleum supply is imported, there's a strong incentive to reverse the growing dependence on foreign oil, much of which comes from the Middle East, a region plagued with political and religious turmoil.
Using hydrogen as an energy carrier in place of fossil fuels—in a fuel-cell-powered vehicle, for example—could alleviate some of those problems, especially if scientists could find a way to produce hydrogen from nonfossil feedstocks. But regardless of how this lightest gas of all is produced, there is no infrastructure in place to supply motorists with hydrogen conveniently and safely. There also is no standard way of storing hydrogen in a car's fuel tank.
All pieces of the hydrogen puzzle need to fit together properly for the gas to be accepted widely as a transportation fuel. But storage issues are unique because they are colored by consumers' needs and expectations, which are guided by the performance, driving range, and comfort of today's gasoline- and diesel-powered cars.
"The goal is to come up with an on-board hydrogen-storage system that provides a driving range of at least 300 miles between fill-ups without compromising passenger or cargo space," says Sunita Satyapal, hydrogen-storage team leader of the Department of Energy's Hydrogen, Fuel Cells & Infrastructure Technologies Program. She explains that achieving that driving range, which is similar to the range of today's conventional cars, would require filling up with roughly 5 to 10 kg of hydrogen. Those values are just a few of the specifications on a detailed list of hydrogen-storage targets developed by DOE in conjunction with automobile manufacturers.
DOE's targets for 2010 call for fuel systems that can store 6% by weight of hydrogen and 45 kg of hydrogen per cubic meter. DOE has set even higher goals for 2015: 9 wt % of hydrogen and 81 kg of hydrogen per cubic meter.
George J. Thomas, a retired Sandia National Laboratories scientist and a recent DOE hydrogen consultant, stresses that the target values represent the hydrogen capacity of the entire fuel system. The system includes the tank, valves, regulators, and other hardware, not just the hydrogen-storage material. Meeting the 6 wt % system goal will mean coming up with a storage medium that can provide roughly 8-12% hydrogen by weight, depending on the type of material and fuel system, Thomas says.
One way to carry a lot of hydrogen is to compress the gas to high pressure. International safety certifications have been issued for lightweight carbon-fiber-reinforced gas cylinders designed to hold hydrogen at 10,000 psi. Satyapal points out that gas-storage systems of that type and of related designs have proven useful in ongoing tests of hydrogen-fueled demonstration vehicles. But in general, she says, compressed-gas technology is unable to pack enough hydrogen into a gas tank to satisfy long-term hydrogen-storage goals for a broad range of automobiles. The volumetric capacity goal (81 kg/m3) can't even be met with liquid hydrogen, which has a density of approximately 70 kg/m3 (at 20 K and 1 atm). Accordingly, most of the research in this area focuses on storing hydrogen more densely, and that means storing the gas in solid materials.
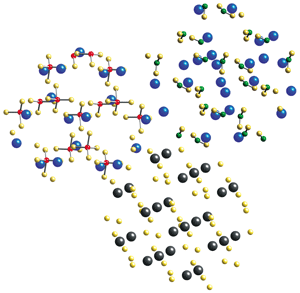
By reacting LiNH2, LiBH4, and MgH2, each of which is shown here as a crystal cross section, researchers have formed a novel composite hydrogen-storage material. Lithium is blue; nitrogen, green; hydrogen, yellow; boron, red; magnesium, black.
Boranes are one example of hydrogen-rich solids that have attracted considerable attention. A number of scientists have focused on ammonia borane (NH3BH3) because hydrogen accounts for nearly 20% of that compound by weight and the solid is neither flammable nor explosive.
Several years ago, researchers reported that roughly 12 wt % of hydrogen can be released by ammonia borane through direct thermal decomposition of the solid at approximately 150 °C. More recently, scientists have examined a variety of strategies for lowering the release temperature and improving other properties of the system. University of Pennsylvania chemistry professor Larry G. Sneddon and coworkers found that the extent and rate of hydrogen release are significantly increased when ammonia borane is dehydrogenated in ionic liquids (J. Am. Chem. Soc. 2006, 128, 7748). And R. Tom Baker and coworkers at Los Alamos National Laboratory, in New Mexico, observed similar enhancements in hydrogen release by mediating the reaction with nickel-based catalysts (J. Am. Chem. Soc. 2007, 129, 1844).
A new twist in the story was announced a few weeks ago. A large research team including Ping Chen of the National University of Singapore; Thomas Autrey at Pacific Northwest National Laboratory in Richland, Wash.; William I. F. David at the Rutherford Appleton Laboratory in the U.K.; and their coworkers converted ammonia borane to lithium amidoborane (LiNH2BH3) and found that the amido compound releases nearly 11 wt % of hydrogen at just 90 °C (Nat. Mater., DOI: 10.1038/nmat2081). In addition, the amidoborane releases hydrogen that's free from borazine, an impurity and fuel-cell poison that evolves from ammonia borane. Now, Autrey says, the strategy is to use additional chemical alterations to further tune the materials' thermodynamic and kinetic properties.
Other researchers have adopted the same strategy—modifying familiar hydrogen-storage materials to improve their properties. In one example involving hydrides, researchers at Ford Motor Co., Dearborn, Mich.; UOP, Des Plaines, Ill.; and the University of California, Los Angeles, prepared a three-component composite by reacting lithium amide, lithium borohydride, and magnesium hydride in the solid state.
The group, which includes Ford chemists Jun Yang, Andrea Sudik, and Don Siegel, found that the temperature at which the ternary composite releases hydrogen is some 50 to 200 ??C cooler than the corresponding temperatures for the individual components and binary composites. Furthermore, the hydrogen is ammonia-free, unlike the gas evolved from some of the binary materials (Angew. Chem. Int. Ed. 2008, 47, 882).

Sorting through the reaction mechanism was no small feat. The group proposes that the overall hydrogen evolution process is composed of eight fundamental reactions. A key finding of the study is that one of those reactions yields Li2Mg(NH)2, which serves as a product seed (or catalyst) for the subsequent reaction and is the main source of the composite's enhanced kinetic properties.
METAL-ORGANIC FRAMEWORK (MOF) compounds, which are porous crystalline materials composed of metal clusters and organic linkers, are another class of materials that has shined recently under the hydrogen-storage spotlight. UCLA chemistry professor Omar M. Yaghi and coworkers showed that one such material, MOF-177, can store 7.5 wt % of hydrogen with a volumetric capacity of 32 kg/m3 at 77 K (J. Am. Chem. Soc. 2006, 128, 3494). And Jeffrey R. Long of UC Berkeley and coworkers reported that a related MOF compound can store 6.9 wt % of hydrogen at 77 K, which, for that material, corresponds to 60 kg/m3 (J. Am. Chem. Soc. 2006, 128, 16876).
It would be nice if someone would wave a magic wand and suddenly make the ideal hydrogen-storage material appear out of thin air. But that's not an option for solving big, real-world problems like coming up with sustainable energy technologies.
"There certainly has been a lot of progress in the past few years," Satyapal notes, but the long-term hydrogen-storage goals have not yet been met. Autrey concurs. "Recently, we've seen lots of singles and a few doubles," he says. "But no one has hit the home run yet."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter