Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Cipla Tests Indian Patent Law
Major generics producer starts selling a Roche cancer drug
by Jean-François Tremblay
January 28, 2008
| A version of this story appeared in
Volume 86, Issue 4
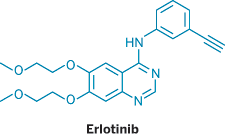
THE DELHI HIGH COURT last week started hearing Roche's demand for an injunction against the Indian firm Cipla, which is marketing a generic version of Tarceva, a Roche lung cancer drug. The case marks the first time that an Indian producer of generic drugs has started local sale of a drug that is under patent protection in that country. Roche received an Indian patent for Tarceva in 2007.
Cipla, India's leading generic drug company in terms of market share, is selling its own low-cost version of erlotinib, the generic name of Tarceva, under the trade name Erlocip. Cipla sells its copycat drug for about $42 per tablet, versus $125 for the Roche version. The drug needs to be taken once daily.
Alexander Klauser, a spokesman for Roche in Switzerland, says the company is seeking an injunction against Cipla largely as a matter of principle. "Just as we respect other people's intellectual properties, we expect ours to be respected," he says. Klauser adds that pharmaceutical patents lie at the root of the system that allows medical innovation to take place. Roche won't provide Tarceva's sales figures in India.
Yusuf K. Hamied, Cipla's chairman, contends that Roche's patent is invalid in India because the patent application for the drug was originally filed in 1995.
In 2005, India started implementing a new patent regime that grants protection to companies that filed applications for their pharmaceuticals in 1995 or later. This move was in keeping with its obligations as a developing country under the international agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), a World Trade Organization-sanctioned trading treaty.
However, WTO had just been formed in 1995 and "countries were given a one-year grace period to implement the TRIPS agreement," Hamied says. Thus, Cipla contends, only those patents filed in India after Jan. 1, 1996, are valid.
Although research on erlotinib had been going on for a long time, it was only in 2004 that the U.S. FDA approved its use in the treatment of lung cancer. Roche launched Tarceva in India in 2006 before it obtained a patent.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter