Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Choosing Metals
The cellular location in which an enzyme folds helps it select the right metal catalyst
by Sarah Everts
October 27, 2008
| A version of this story appeared in
Volume 86, Issue 43

THE EXQUISITE OPERATION of many enzymes relies on slipping the correct metal ion into the protein's active site, yet the mechanism by which proteins choose one ion over another—say, copper over manganese—has long befuddled researchers. Now biochemists from Newcastle University, in England, led by Nigel Robinson, report that cells can ensure the enzyme picks the right metal ion by controlling which cellular compartment the protein folds in.
"This research is really exciting," comments Valeria Culotta, a toxicologist at Johns Hopkins University. "How a protein picks the right metal among a sea of metal ions in the cell" has been a challenging question because some metal ions are more abundant or have stronger binding affinities, she says. For example, copper binds to proteins more strongly than do nickel, cobalt, iron, and manganese, but the latter trace metals are essential for the function of many enzymes.
About a decade ago, Culotta and others discovered chaperones that place copper in the active site of some proteins. Since then, scientists have looked for other trace metal ion chaperones with only limited success. Other researchers have shown that the orientation of amino acid side chains in an enzyme's active site can help the protein nab the right ion, but this theory can't entirely account for ion selectivity.
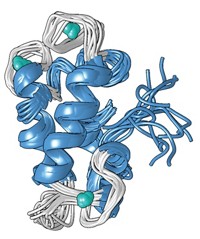
Recently, Robinson and his colleagues found in a cyanobacterium two proteins, CucA and MncA, that require copper and manganese, respectively, to be catalytically active. Strangely, both proteins used one glutamate and three histidine residues, in exactly the same orientation, to hold their catalytic metal ions in place (Nature, 2008, 455, 1138).
The complementary ion coordination was fortuitous, Robinson says, because it permitted his team to investigate how one protein incorporates copper while the other protein incorporates manganese, despite the fact that both proteins prefer copper 10,000 times more.
It turns out that the cell places the correct metal in the protein by controlling where the nascent protein folds. Although both proteins are produced in the cytoplasm of the cell, only the manganese-containing protein is allowed to fold there. In the cytoplasm, the concentration of high-binding-affinity copper is negligible, allowing the manganese to slip into place.
Meanwhile, the copper-containing protein is not allowed to fold in the cytoplasm, but is instead transported to an outer compartment in the bacterial cell called the periplasm, where there are higher concentrations of free copper that the protein can incorporate into its active site.
"It's as if evolution hasn't attempted to produce a protein that preferentially binds manganese, but instead solves the problem of selective metal ion incorporation by changing the folding location," Robinson says.
Even though higher organisms, like humans, don't have a periplasm, we do have far more cellular compartments, called organelles, than bacteria, Culotta notes. "So there are probably a lot more examples of this metal ion selection in nature," she adds.
Robinson and his colleagues hope their results will inform synthetic biologists, who may want to control the placement of catalytic ions in engineered proteins. The work also furthers the understanding of "how cells do inorganic chemistry and sets the next stage for understanding pathologies that arise when metal trafficking in the cell goes awry," adds Thomas V. O'Halloran, a chemist who studies metalloproteins at Northwestern University.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter