Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
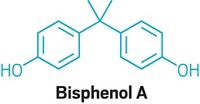
Bisphenol A Battle
As new information trickles in, drop by drop, the debate over the estrogenic plastics chemical intensifies
by Britt E. Erickson
November 17, 2008
| A version of this story appeared in
Volume 86, Issue 46
WHEN THE NEW Administration takes over in January, one of the first issues the Food & Drug Administration will need to deal with is bisphenol A (BPA). The controversial plastics chemical has been at the center of a contentious debate since last spring because of health concerns associated with its use in consumer products such as baby bottles and infant formula cans (C&EN, June 2, page 36).
The battle over BPA, a known endocrine disrupter, has intensified in recent months. As a result, manufacturers of infant formula are now voluntarily phasing out BPA from their packaging, and the canned food industry is under pressure from advocacy groups to do the same. Meanwhile, FDA is still deciding what action to take.
At the end of October, a subcommittee of FDA's Science Board released a report calling the agency's risk assessment of BPA inadequate (C&EN, Nov. 3, page 12).
In response to the report, FDA said in a statement that it recognizes "the uncertainties raised in some studies" and that "additional research would be valuable." The agency added that it is "moving forward with planned research to address the potential low-dose effects of bisphenol A." The agency has not said whether it will redo the assessment as recommended by the subcommittee.
At about the same time that the subcommittee report was released, a group of 36 scientists published a commentary in Environmental Health Perspectives, the peer-reviewed journal of the National Institute of Environmental Health Sciences, identifying flaws in the criteria FDA used to select which studies it included in its BPA analysis (Environ. Health Perspect., DOI: 10.1289/ehp.0800173).
Just weeks before the BPA subcommittee presented its report to FDA's Science Board, Martin Philbert, chairman of the subcommittee and professor of environmental health science at the University of Michigan, came under fire for not disclosing a potential conflict of interest related to a $5 million donation that his center at the University of Michigan received from a former medical device manufacturer and outspoken BPA proponent (C&EN, Oct. 20, page 10). FDA cleared Philbert of any wrongdoing a few days before the Science Board met to discuss his subcommittee's report.
Several groups that initially called for FDA to remove Philbert from the BPA subcommittee and perform a new review because of the appearance of a conflict of interest changed their tune when they saw that the subcommittee's report heavily criticized FDA's draft BPA assessment. This flip-flop in calling for a new study does not sit well with some stakeholders.
The process "cannot be deemed 'legitimate' only if it yields the result desired by those who cried foul earlier this week," John M. Rost, chairman of the North American Metal Packaging Alliance, told FDA's Science Board at its October meeting. "As a concerned stakeholder, NAMPA believes the public trust will be further eroded if all parties do not demand better." NAMPA represents the metal can industry.
"Liquid formula is the biggest culprit in exposing infants to a toxic hormone-disrupting chemical."
At the Science Board meeting, Philbert presented the main criticisms highlighted in the subcommittee's report. He pointed to the inadequacy of the infant formula samples used in FDA's assessment, saying there were not enough of them, they were too old, and they were not geographically representative.
In addition, Philbert emphasized the lack of data on nondietary-related sources of BPA exposure. "FDA did not look at the totality of BPA exposures, so it is difficult to assess where in the range of exposures food contact applications lie," he told the Science Board.
ANOTHER PROBLEM with the assessment, Philbert said, is the lack of justification for inclusion or exclusion of scientific studies. FDA has been criticized by several groups for relying too heavily on one industry-funded study that followed good laboratory practices (GLP). "Non-GLP studies should not necessarily be excluded," Philbert emphasized at the meeting.
GLP studies tend to use larger numbers of animals and have more rigorous reporting and characterization, but that doesn't mean that non-GLP studies should be ignored, Philbert added. Non-GLP studies that were judged adequate by the National Toxicology Program's Center for the Evaluation of Risks to Human Reproduction)—a group that reached a different conclusion in September than FDA did about the safety of BPA (C&EN, Sept. 8, page 28)—should have been included, he stressed.
As FDA's methods for determining the safety of BPA are called into question, the agency continues to assure the public that exposure to low levels of BPA via food packaging is safe. In response to the Science Board report, the agency released a statement saying, "The present consensus among regulatory agencies in the United States, Canada, Europe, and Japan is that current levels of exposure to BPA through food packaging do not pose an immediate health risk to the general population, including infants and babies."
On Oct. 17, Canada became the first country to ban polycarbonate baby bottles and set limits on how much BPA can migrate from infant formula cans (C&EN, Oct. 27, page 8). FDA said its Canadian counterpart, Health Canada, acted "out of an abundance of caution." But Health Canada's assessment reached the same conclusion as FDA did that BPA from food packaging "is not expected to pose a health risk to the general population, including newborns and infants," according to FDA.
The chemical industry points out that BPA has been used in consumer products for more than 50 years and is an essential ingredient in the epoxy resins that keep bacteria out of canned foods. But it says it will comply with whatever FDA decides in its final assessment. "If the agency determines that existing margins of safety are insufficient in infant applications, our member companies that manufacture BPA will put processes in place to promptly phase out the use of materials containing BPA in baby bottles and infant formula packaging," the American Chemistry Council, a trade group that represents chemical manufacturers, said in a statement.
IN PUBLIC COMMENTS delivered during the FDA Science Board meeting in October, Mardi Mountford, executive vice president of the International Formula Council, a trade group representing infant formula manufacturers, said, "We have continued to work with our suppliers to identify opportunities for packaging without BPA. There are no quick solutions, and in the interest of safety and consumer confidence, any new alternatives need to be carefully assessed to ensure the highest possible standards of quality."
Manufacturers will have to work fast because many U.S. infant formula manufacturers also sell their products in Canada, Sonya Lunder, a senior analyst with the nonprofit Environmental Working Group, told reporters during an EWG press briefing. The briefing was held just hours before FDA's Science Board met to discuss the flaws in FDA's draft assessment. Infants in the U.S. could thus get protection because of more restrictive Canadian regulations, she added.
FDA continues to assure the public that exposure to low levels of BPA via food packaging is safe.
In a recent Canadian study, analysts found BPA that had leached from packaging in every brand of liquid infant formula tested, Lunder said. The study "found significant differences in brands based on the thickness of the epoxy lining used in these liquid formula cans and found no detectable BPA in powdered formula," she added. EWG is asking manufacturers of liquid formula to package their products in plastic instead of metal cans.
And the group isn't stopping there. It is also going after the canned food industry, asking major manufacturers to voluntarily remove BPA from their products. "Liquid formula is the biggest culprit in exposing infants to a toxic hormone-disrupting chemical, but kid-friendly foods like canned chicken noodle soup and ravioli also have high levels of BPA," Lunder said.
When EWG tested canned foods in 2007, the group detected BPA in 57% of the samples, Lunder noted. "The highest concentrations were in canned pasta, soup, tuna fish, and some vegetables," she said.
MEANWHILE, new studies on the health effects of BPA continue to appear in the scientific literature. Health concerns have gone beyond the potential reproductive and developmental effects of BPA in infants and children and now include the possibility that BPA plays a role in cardiovascular disease and type 2 diabetes in adults (C&EN, Sept. 22, page 14). BPA has also been shown to interfere with chemotherapy in breast cancer patients. FDA's Science Board recommended that the agency include these new studies in its final assessment.
Some people are worried that a new BPA assessment from FDA could take years. "My concern now is that FDA's response will be, 'Let's do more research and come back in five years to see if everyone agrees.' That would be a profoundly misguided action," John Peterson Myers, founder and chief executive officer of Environmental Health Sciences, an advocacy group, said during the EWG press briefing. "There was enough science even before FDA began its assessment to conclude that BPA doesn't meet FDA's safety standard," Myers noted.
In some cases, people who are tired of waiting for federal action have taken the situation into their own hands. Last spring, amid pressure from concerned consumers, several retailers began voluntarily removing products containing BPA from store shelves. Baby bottle and reusable water bottle manufacturers began producing BPA-free alternatives. And under pressure from Congress and state attorneys general, infant formula manufacturers said they would begin phasing out BPA from their packaging.
Although some states are working toward regulating BPA, thus far none has been successful. For example, a California bill that would have banned BPA from baby bottles and other products intended for children was defeated at the end of August.
"One of the main factors in the defeat was that just days before the vote, FDA released this now-discredited report. We were suspicious at the time that the release was timed to influence the California debate and was done at the behest of the food companies," Kenneth Cook, president and cofounder of EWG, said during the group's press briefing. EWG intends to push California legislators to reintroduce that legislation, he added.
As the BPA battle continues, it is likely to result in more than just potential regulations. It may also drive big changes at FDA. President-Elect Barack H. Obama is expected to take a close look at FDA's ability to protect public health, and the House Committee on Energy & Commerce has already been investigating FDA for more than a year. These factors are leading many observers to predict that FDA will be overhauled by the next Congress.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter