Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Scrutinizing Catalysts
New methods reveal details hidden by conventional analyses
by Mitch Jacoby
November 17, 2008
| A version of this story appeared in
Volume 86, Issue 46

TWO NEW METHODS for probing solid catalysts are revealing chemical and physical details typically inaccessible to conventional analytical techniques. The information they yield provides insights into how particles' surfaces change while they mediate chemical reactions and uncovers key differences between seemingly identical catalyst particles. Such information could be used to design new catalysts with improved efficiency, durability, and product selectivity.
A team led by Frank M. F. de Groot and Bert M. Weckhuysen of Utrecht University, in the Netherlands, devised an analytical procedure based on scanning transmission X-ray microscopy. The team used the technique to identify numerous chemical species on the surface of an iron Fischer-Tropsch catalyst and to prepare chemical maps that pinpoint the species' locations with 15-nm resolution (Nature 2008, 456, 222). Fischer-Tropsch chemistry is a surface polymerization used commercially to make fuels from a mixture of CO and hydrogen derived from natural gas or coal.
The X-ray system includes a microreactor that operates at atmospheric pressure and temperatures up to 350 ºC. The device probes catalyst surfaces not only before and after reactions, as in conventional analyses, but also in situ, that is, during reactions, as short-lived species are formed on the surfaces.
The group observed that before the iron in the catalyst is exposed to the reactive gases, the metal, which is supported on SiO2, is present mainly in the α-Fe2O3 form. Not long after the reaction starts, Fe2O3 gives way to various species including Fe3O4, Fe2SiO4, metallic iron, and iron carbide. In addition, the surface becomes coated with hydrocarbon Fischer-Tropsch products.
Compared with transmission-electron and scanning-probe microscopies, the X-ray method has lower spatial resolution. Yet it provides greater chemical detail and is compatible with higher pressures of reactive gases. In contrast, optical microscopy and infrared spectroscopy can reveal chemical detail but generally with lower spatial resolution.
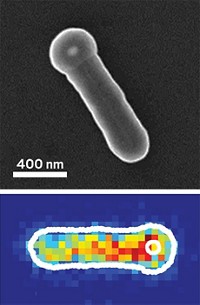
Meanwhile, Peng Chen and coworkers at Cornell University have developed a procedure for using fluorescence microscopy to monitor individual gold nanoparticles as they catalyze reactions one molecule at a time. Specifically, the group probed the solution-phase catalytic reduction of resazurin molecules to resorufin by measuring the fluorescent light bursts that mark each catalytic event (Nat. Mater., DOI: 10.1038/nmat2319).
Using the single-particle/single-molecule method, the team made several observations that would have been hidden by conventional methods, which measure averaged values. For example, they determined that despite the gold nanoparticles' uniform size and composition, the particles exhibit key differences in selectivity and widely varying catalytic activity levels, which fluctuate in a time-dependent way as a result of surface restructuring.
"These are both very impressive studies," says Texas A&M University chemistry professor D. Wayne Goodman. State-of-the-art techniques such as these can be used to address an inventory of long-standing problems in catalysis, he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter