Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
New Role For Transferrin
Blood protein forms fibers, releases rust
by Rachel Petkewich
February 18, 2008
| A version of this story appeared in
Volume 86, Issue 7
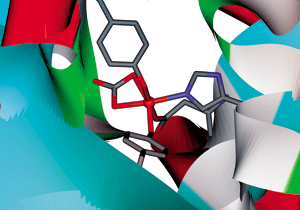
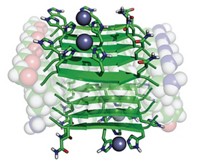
Transferrin, the blood protein responsible for transporting iron throughout the body, can assemble into fibers that release bits of rust (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200705723). These unexpected findings from in vitro studies may help researchers decipher iron's role in various neurodegenerative diseases.
Diseases such as Parkinson's, Alzheimer's, and Huntington's have been associated with the defective regulation of iron in the brain. "If transferrin plays a role in accumulation of iron in brain tissue, then understanding the mechanism of this process will allow the design of drugs that could disrupt transferrin aggregation," says Peter J. Sadler, a chemistry professor at the University of Warwick, in England. Sadler carried out the work in collaboration with chemistry professor Sandeep Verma of the Indian Institute of Technology, Kanpur, and colleagues.
Their findings have intrigued other researchers. "Transferrin has never been shown to form fibrils, let alone ones with pockets of mineralized iron," says N. Dennis Chasteen, a chemistry professor at the University of New Hampshire. "Until this paper, human serum transferrin had been universally thought of simply as a transport protein for iron and other metals in blood circulation."
Transferrin is best known for transporting two Fe3+ ions. Each ion is tightly bound to four amino acid side chains that form a compartment and a carbonate anion that acts as a synergistic keystone.
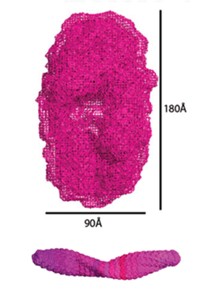
Sadler and his colleagues deposited dehydrated transferrin on various surfaces under conditions that mimic living systems. They used microscopy techniques to show that the protein assembles into fibers and forms metal nanocrystals. Diffraction patterns indicate similarities in composition between the crystals and an iron oxide mineral called lepidocrocite. The researchers plan further experiments to see whether transferrin fibers form and iron minerals accumulate in vivo.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter