Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Arthur C. Cope Scholar Awards
by Linda Raber
February 25, 2008
| A version of this story appeared in
Volume 86, Issue 8
Colin Nuckolls, 38, is "redefining the field of physical organic chemistry," according to his Columbia University colleague and Ph.D. adviser, Thomas J. Katz. Katz says Nuckolls combines three talents: "one, an exceptional ability to carry out the physical studies required to analyze and refine the properties of materials; two, a superb imagination for molecules that should have significant new properties; and three, a deep knowledge of organic synthesis."
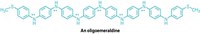
The first project Nuckolls worked on as a faculty member at Columbia centered on the design of columnar liquid crystals wherein intermolecular correlations were enforced by hydrogen-bonding interactions between amide groups. The goal was to create polar organization along the axis of the column. According to Massachusetts Institute of Technology professor Timothy Swager, "The work Colin performed was elegant in conception, well-executed, and has contributed to the design of motifs that can be used to create macroscopic dipoles at interfaces." Swager says he expects that these methods will be important for the separation of charge required to properly mimic nature's photosynthetic processes.
More recently Nuckolls has burst onto the field of molecular electronics by pioneering preparation of molecule-sized electrical circuitry. His syntheses of new organic chemical structures and his investigations of their self-assembly, interactions with surfaces, and electrical transport properties have resulted in a number of scientific insights and have significantly changed the ways in which the electronic properties of single molecules can be examined.
For example, one set of studies greatly enhanced the ways that electronic transport between organic molecular wires and surfaces could be studied. Previously, almost all such research has relied on the interaction of a thiol and gold to link the wire to the surface. Nuckolls, however, was able to grow single-molecule wires, by means of the olefin metathesis reaction, from ruthenium surfaces that had been functionalized with monolayers of carbenes. Another method he developed uses the undercoordinated metal atoms at the ends of metal electrodes to bind specifically to amino and phosphino end groups on conjugated molecules.
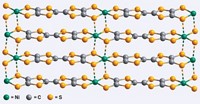
Another of Nuckolls' studies was based on the idea that it should be possible to oxidatively cut a molecule-sized gap in a nanotube that is connected to two electrodes and then bridge the gap by covalently bonding synthesized organic conductors to the carboxylic acid functions at the ends of the gap. The result was a synthesis that gives reliably better yields of single-molecule electrical devices that have two significant virtues. One is the high conductance found in these molecular circuits-approaching one-tenth the fundamental quantum of conductance-and the other is sensitivity to the surrounding environment.
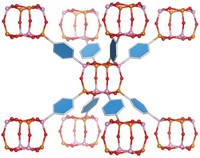
Although single-molecule conductors have previously been prepared and analyzed by various and difficult methods, Nuckolls' method is easy and general. It has allowed him not only to discover the devices described above, but also to discover a principle controlling self-assembly. Although it might have been thought that self-assembly, and therefore conductivity, would be enhanced by molecular planarity, Nuckolls found that -by synthesizing many novel organic structures and measuring their conductive properties in the gap created by incising nanotubes-a nonplanar hexabenzocoronene had the best field effect properties of any columnar mesogen. Nuckolls thus arrived at the hypothesis that contortion, not planarity, can cause molecules to fit together particularly well and can thereby endow their assemblies with superior conductive properties.
Working with adviser Marye Anne Fox, Nuckolls received a B.S. in chemistry from the University of Texas, Austin, in 1993. In 1998, he received a Ph.D. under the mentorship of Katz. A postdoctoral stint followed from 1998 until 2000 with Julius Rebek Jr. at Scripps Research Institute, in La Jolla, Calif. He joined the faculty of Columbia University in 2000 as an assistant professor. He has been full professor there since 2006.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter