Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Long-Sought Benzenelike Molecule Created
Aromaticity of organic-inorganic hybrid resembles benzene's
by Stu Borman
January 5, 2009
| A version of this story appeared in
Volume 87, Issue 1
AN ELUSIVE organic-inorganic hybrid compound that is related to benzene electronically and structurally has been synthesized for the first time, making possible analysis of its properties.
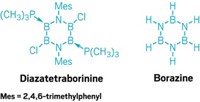
The heterocyclic compound, 1,2-dihydro-1,2-azaborine, is isoelectronic with benzene—that is, it has the same number of valence electrons and the same atomic connectivity as benzene, although some of its atoms are different. Its synthesis could help improve scientists' understanding of heterocycle aromaticity and could lead to new molecules and materials for biomedical and materials science applications.
The quintessential aromatic organic compound benzene owes its superstability to its specific system of conjugated double bonds. Borazine, an inorganic boron-nitrogen compound that is isoelectronic with benzene, is also aromatic, but to a lesser extent.
For decades, chemists have been trying to synthesize 1,2-dihydro-1,2-azaborine, a carbon-boron-nitrogen compound with a structure that is intermediate between benzene and borazine. A number of 1,2-azaborines have been made, but the parent compound, 1,2-dihydro-1,2-azaborine, had eluded assembly.
Now, this synthesis has been achieved by synthetic organic-organometallic chemist Shih-Yuan Liu of the University of Oregon, Eugene; computational chemist David A. Dixon of the University of Alabama, Tuscaloosa; and their coworkers (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200805554).
Attempts to synthesize 1,2-dihydro-1,2-azaborine go back some 40 years to efforts by the late theoretical chemist Michael J. S. Dewar, who helped found the research area of nonbenzenoid aromaticity. Previous attempts by Dewar's group and others to create the compound by dehydrogenation required high temperatures that prevented it from forming, Liu notes. "We developed a synthetic strategy that avoided high temperatures," he says. "The key to our success was finding a suitable N-protecting group—tert-butyldimethylsilyl allyl amine—which was stable toward the acidic conditions required for assembly of the aromatic heterocycle yet could be readily removed after the B–H group had been installed."
Liu, Dixon, and coworkers find that the compound's aromaticity resembles benzene's in two ways. Not only is its aromatic stabilization energy of 21 kcal/mol intermediate between that of benzene (34 kcal/mol) and borazine (10 kcal/mol), but it also smells like benzene. The researchers detected the smell serendipitously when they were measuring the compound's infrared spectrum.
Organometallic chemist Arthur J. Ashe III of the University of Michigan, Ann Arbor, the modern pioneer of 1,2-azaborine synthesis, comments that "the imaginative synthesis of the parent compound can be regarded as the climax" of recent azaborine studies. "It allows this close relative of benzene to be studied in detail. The lack of perturbing substituents should allow focus on the intrinsic properties of the parent ring," Ashe says.
The work could aid the synthesis of boron-based pharmacophores, markers, and neutron-capture agents for biomedical use and boron-based optoelectronic and hydrogen-storage compounds for materials science applications.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter