Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Peeking More Closely At Gold Catalysis
Chemists have learned more about two types of intermediates—cationic and carbene—that participate in gold catalysis
by Carmen Drahl
March 9, 2009
| A version of this story appeared in
Volume 87, Issue 10

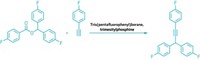
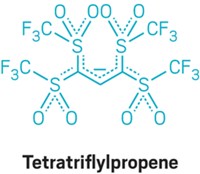
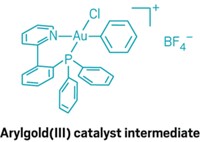
By employing a cyclopropene rearrangement reaction, chemists have uncovered more about the types of intermediates that participate in gold catalysis. Researchers often invoke electron-poor intermediates such as carbenes or cations to explain the mechanisms of gold catalysis, but such intermediates have tended to elude characterization. Günter Seidel, Richard Mynott, and Alois Fürstner of the Max Planck Institute for Coal Research, in Mülheim, Germany, report their efforts to generate gold carbenes by opening a disubstituted cyclopropene with a gold catalyst (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200806059). This type of rearrangement has been used to characterize carbene complexes of many transition metals. However, NMR spectra of the team's reactions were more consistent with a cationic species than a carbene. The authors caution that the oxygen atoms used to stabilize the intermediates could bias their system toward cationic forms. Nonetheless, these results, together with their prior reactivity data (Angew. Chem. Int. Ed. 2008, 47, 5030), lead them to conclude that evidence for cationic behavior should be considered when evaluating mechanisms for gold catalysis. The German team's results add to recent definitive observations of the two intermediate types by independent teams (J. Am. Chem. Soc. 2008, 130, 17642; Organometallics 2009, 28, 666).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter