Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Eyeing Electrons
Laser pulse enables tracking of electron distribution in reactions
by Jyllian N. Kemsley
March 16, 2009
| A version of this story appeared in
Volume 87, Issue 11

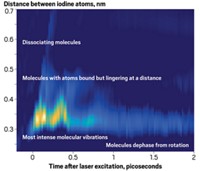
BY USING LASER PULSES to align molecules for just a few picoseconds, researchers can now study how electron rearrangement influences chemical reactions, reports a group led by Albert Stolow of the Canadian National Research Council's Steacie Institute for Molecular Sciences (Science 2009, 323, 1464).
Scientists have used femtosecond spectroscopy for years to follow atoms in chemical reactions. The random orientation of molecules in the gas phase, however, can make it difficult to tease out what's happening to the electrons in such reactions. For example, when a molecule is ionized, scientists would like to use the energy and direction of the emitted electron to determine the shape of the molecular orbital from which it came. But if molecules are oriented randomly, the emission directions are blurred.
Separately, scientists had also previously used ultrashort laser pulses to align molecules temporarily for optical or mass spectrometry experiments. Stolow and colleagues now take that one step further to combine alignment with pump/probe femtosecond laser spectroscopy for photoelectron detection.
"The clever extension in this report to use photoelectron detection is a welcome contribution," says Ahmed H. Zewail, a professor of chemical physics at California Institute of Technology. Zewail received the Nobel Prize in Chemistry in 1999 for pioneering the use of femtosecond spectroscopy to study chemical reactions.
Stolow and colleagues demonstrated the method by using a femtosecond laser pulse to push molecules of carbon disulfide (CS2) into alignment. The molecules stay put for just a few picoseconds, but that's long enough to use time-resolved photoelectron spectroscopy (TRPES) to ionize the aligned molecules and examine what happens as they dissociate. Stolow likens the power of TRPES with alignment versus without it to that of single-crystal X-ray crystallography versus powder diffraction.
"You can see the angular distribution of electrons changing and directly relate it to the way the molecular orbitals are changing during a chemical reaction," Stolow says. "Now we can see not just how atoms are moving but how electrons are arranging in concert with the atoms."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter