Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Faster-Charging Batteries
Method creates lithium-ion battery that charges and discharges in seconds
by Elizabeth K. Wilson
March 16, 2009
| A version of this story appeared in
Volume 87, Issue 11

BY MERELY TWEAKING a synthetic strategy for making a popular lithium-ion battery material, scientists have produced a battery that charges and discharges in a matter of seconds, rather than the minutes needed for typical lithium-ion batteries.
Used in cell phones, laptops, and other devices, Li-ion batteries are attractive because they can hold a lot of charge, yet they're relatively slow to put it out or take it up. The new battery's swift charging and discharging could help make possible the use of Li-ion batteries in applications such as electric cars.
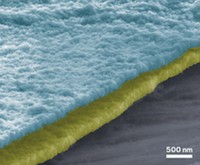
To build the battery, materials science professor Gerbrand Ceder and graduate student Byoungwoo Kang at MIT synthesized a popular modern Li-ion battery material, LiFePO4 (Nature 2009, 458, 190).
The group used standard starting materials for preparing LiFePO4, but in slightly different amounts than usual. This method produced 50-nm-sized LiFePO4 particles, each coated with a glassy substance that's slightly depleted in iron and phosphorus atoms relative to the LiFePO4 bulk material.
Recent theoretical and experimental studies from several labs have shown that lithium ions travel speedily through battery material itself, but that the ions' ability to move across a surface—which depends on the arrangement of atoms on the surface—and into the bulk material may be the bottleneck.
The coating on their material, Ceder and Kang believe, solves that problem: The configurations of Li, O, and P atoms on the material's surface provide ready pathways for Li to migrate in and out of the surface rapidly.
"This is an important development because it provides a simple and inexpensive synthetic route to a high-quality active material for a well-established electrode system," says Thomas Richardson, a chemist and battery researcher at Lawrence Berkeley National Laboratory.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter