Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Rotaxanes Go Hybrid
New compounds combine inorganic and organic parts
by Bethany Halford
March 23, 2009
| A version of this story appeared in
Volume 87, Issue 12

THE HYBRID TREND isn't just for automobiles anymore. Now, molecular machines are taking up the fashionable hybrid label, thanks to a new type of interlocked organic-inorganic molecule synthesized by chemists in the U.K. (Nature 2009, 458, 314). Combining two distinct chemistries into one molecule could expand the realm of applications for these systems in areas such as quantum computing.
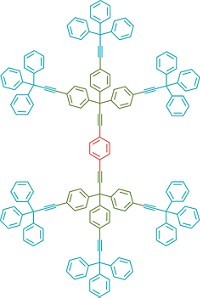
The new hybrid molecules are rotaxanes—bead-on-a-string-type structures in which a linear molecule threads through at least one cyclic molecule. Bulky components at each end of the linear molecule act as stoppers to keep the compound from coming apart. A team led by David A. Leigh of Scotland's University of Edinburgh and Richard E. P. Winpenny of England's University of Manchester built hybrid rotaxane structures from linear secondary amine threads and heterometallic rings.
Although chemists previously have made rotaxanes that incorporate or bind to metal ions, "this is the first example of a discrete molecule in which wholly organic and essentially inorganic components are linked mechanically at the molecular level," Leigh says. One of the hybrid rotaxanes the group synthesized acts as a molecular shuttle, wherein the ring can move between two ammonium binding sites on the thread.
"Inorganic and organic structures are often associated with rather different types of chemistries: metal-based magnetism, electronic properties, and catalysis, on the one hand, and structurally, functionally, and dynamically complex organic molecules on the other," Leigh notes. "The combination of both types of structural unit in a single molecule—particularly one in which the components can move with respect to each other—leads to hybrid structures with characteristics and properties of both chemistries and perhaps some new property traits altogether."
"I must say that I find the results very exciting," comments Jean-Pierre Sauvage, a pioneer in the field of mechanically interlocked molecules who teaches chemistry at Louis Pasteur University, in Strasbourg, France. "It could be envisioned that, one day, these frameworks could be set in motion by sending a given signal to the molecular assembly."
To that end, Leigh says that the next step for this project is to synthesize such stimuli-switchable shuttles. The magnetic properties of the inorganic ring make it a promising component for quantum computing systems, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter