Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
REACH Enters A New Phase
Companies worldwide join forces to fill in data gaps for registration of chemicals in Europe
by Patricia L. Short
April 27, 2009
| A version of this story appeared in
Volume 87, Issue 17

AFTER NEARLY A DECADE of acrimonious debate and legislative horsetrading—and a hectic preregistration period last year—the European Union's program for Registration, Evaluation, Authorization & Restriction of Chemical substances (REACH) is well under way. Companies that make and use chemicals have begun registering them in a phased program with an initial deadline of the end of 2010.
And as the gathering of information needed for registration heats up, non-European producers and users of chemicals are working out how they can take an active part in the process. Sitting on the sidelines isn't an option: Although REACH is a European program, it will have a decided impact upon chemical producers around the world.
Already, some court cases have been launched to clarify how the legislation will be interpreted for imports. And anecdotes are circulating about overzealous customs officials demanding preregistration documentation before allowing chemicals and products containing chemicals to enter European ports, a protocol that REACH does not specify as a requirement.
Non-European producers were initially rattled by REACH because only European companies could preregister chemicals. And yet to stay on the market, chemicals needed to be preregistered while information dossiers are generated for registration.
The solution was to have non-European companies appoint "only representatives," or ORs, to preregister on their behalf. An OR might be the EU-based subsidiary of the foreign supplier, an importer, a legal representative of the foreign supplier, or a consultant. Now, ORs are joining with European producers to register their chemicals.
"There is a very short timetable for companies to come together and agree on some very complex issues."
Registrations will be submitted by Substance Information Exchange Forums (SIEFs), which will pool safety and toxicology data into one dossier for each chemical to be registered. Such a combined effort, the thinking goes, will forestall duplicate tests and minimize testing on animals.
Anthony O'Brien, a REACH specialist at U.K.-based Croner Consulting, points out that software developed by the Helsinki-based European Chemicals Agency (ECHA) automatically placed chemicals preregistered with either the same name or the same chemical identifier into a pre-SIEF. Final SIEF formation, however, means that certain questions must be resolved. For example, does glacial acetic acid differ enough from dilute acetic acid to warrant a separate SIEF?
What's more, REACH doesn't end when a chemical is registered, states REACHReady, part of the U.K.'s Chemical Industries Association (CIA), in one of its guidance notes. All the companies involved need to maintain "living" documents about each chemical that, for example, provide updated test data or new exposure details.
ORs have additional duties, CIA points out. For instance, they must contact importers of the chemical in question for updates on use patterns, volumes imported, exposure scenarios, and the like.
SOME U.S. COMPANIES have been able to turn to their European operations to cope with REACH. Rohm and Haas, for one, let its Europe, Middle East, and Africa (EMEA) operations take on the job. "We are actively complying with REACH requirements," says Gray Wirth, head of the EMEA region for Rohm and Haas, which is now part of Dow Chemical. Although his business is based in Switzerland, the country is in the EU-affiliated European Economic Area, where companies are able to preregister chemicals.
So far, the REACH efforts of Dow and Rohm and Haas are separate, and Dow's Swiss-based European headquarters has taken a different approach. Walter van het Hof, Dow's public affairs leader for designed polymers and latex, says the company established an OR "trustee" model, working with consulting firm PricewaterhouseCoopers (PwC), to address business confidentiality issues posed by the supply chain.
According to Hans-Norbert Adams, leader of Dow's office for REACH implementation, customers from the Pacific area and, to a lesser extent, from the U.S. are taking a real interest in REACH, and he thinks it will only increase. As soon as a customer joins Dow's model, he adds, it automatically becomes represented in SIEFs by virtue of Dow's membership.
"One reason we think it will do well," Adams adds about Dow's approach, is that "we are hearing from PwC that other companies are interested in setting up something similar. We have set a precedent."
Companies with European subsidiaries are probably more fortunate than those without because their employees will be in synch with the thought processes that led to the development of REACH.
Firms without a big European presence are at a disadvantage, believes Barbara Vogt, managing director of Tox Focus, a Stamford, Conn.-based toxicology consulting firm that specializes in REACH compliance. "The components of REACH are still very foreign to non-European companies," she says.
Before starting up her consultancy, Vogt worked for what she describes as "a mid-sized U.S. company with a sizable presence in Europe." A toxicologist, she was charged in 2006 to help assess the potential impact of REACH. The company's managers figured the toxicologists would be sending in the reports needed for registration, she recalls.
However, it soon became clear, Vogt says, that REACH would be "a very complex project-management challenge," far beyond just toxicology. REACH will force serious business decisions, she explains, ranging from how distribution will be handled to whether it will be worth the cost of registration to continue carrying certain products at all.
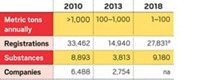
For example, if a chemical is preregistered, it can still be sold in Europe up to a deadline based on sales volume. Higher volume products, those sold in volumes above 1,000 metric tons per year, can be sold through 2010 before full registration is needed. Volumes of between 100 and 1,000 metric tons per year must be registered by June 2013. And lower volume chemicals of 1 to 100 metric tons per year can be marketed through 2018 before they must be registered. Producers must decide whether to proceed with registration or pull the product when the deadline arrives.
Vogt points out another hurdle for chemical registrations. Although ECHA administers REACH, scientific assessment is the responsibility of the various EU countries.
"Each country has their pet chemicals they don't like. So various countries will review the submissions," Vogt warns. Many of the chemicals have been around for a long time, she says, but "as a toxicologist, I think there are still a lot of basic questions that we haven't developed the basic science to answer yet."
SIEFs, for example, will have to resolve conflicting results from different tests. Which tests will be judged more valid? What results will be given more weight? "There is a lot of that disagreement in the field," Vogt says. "We must have the experts gather and come to a consensus about these issues."
Still in the early days of preparing registrations, everyone is on a learning curve, says E. Spencer Williams, a health scientist at San Francisco-based consulting firm Chemrisk.
"We're putting it together as we go along," Williams observes. It is up to the companies to ensure that they are complying with the law, he says, but the approach will depend on the size of the company.
When data gaps are identified in the registration dossier, Williams says, SIEFs will have to decide how to proceed. A SIEF could, for example, hire an external source to conduct needed studies or commission the work in the labs of member companies.
Williams says his biggest concern is that "companies never want to spend the money and the effort until the last minute, until the 11th hour. If companies do wait until the last minute, they may have difficulty finding enough help." The needed technicians, epidemiologists, and similar experts may not be available. "There is a very short timetable for companies to come together in SIEFs and agree on some very complex issues," he points out. "It's a tall mountain to climb in a very short time."
That is particularly true given the number of SIEFs that will be needed.
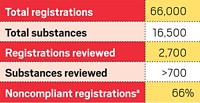
IN JANUARY, at a conference on REACH organized by the Environmental Technologies Center of Industrial Collaboration at England's University of Hull, ECHA senior scientific adviser Derek Knight pointed out that during the preregistration period between June 1 and Dec. 1, 2008, more than 2.7 million preregistrations were submitted—nearly 20 times the original estimate.
According to Knight, ECHA staff have been sorting out duplications, chemicals with multiple or poorly identified names, and so on, to create the lists from which SIEFs will be formed.
ECHA statistics indicate that nearly 146,000 SIEFs will be needed, of which some 15% will have more than 50 members. Two SIEFs will have more than 5,000 members. Such large forums will be "extremely complex to manage," Knight observed. "It will be a huge challenge."
However, it's difficult to argue with a stated aim of protecting human health and the environment, the overarching goal of REACH. And companies that have tried to do so during the years of debate have only hurt themselves and the industry, many observers believe.
At the University of Hull conference, Janet Winter Blaschke, managing director of Manhattan Beach, Calif.-based International Cosmetics & Regulatory Specialists, noted that REACH's very existence reflects a legislative response to pressures from nongovernmental organizations (NGOs) and a hostile public.
"Too often," she said, "industry is seen as irresponsible, and NGOs have government and industry firmly in their sights." Moreover, she pointed out, people in environmental pressure groups "aren't just hippies anymore. They raise valid, science-oriented issues."
Harking back to California's activism in adopting stringent environment standards that eventually rolled throughout the U.S., she noted that the state's legislators are now interested in adopting a California version of REACH.
"It's important for U.S. companies to understand that it's not just the U.S. anymore. U.S. producers have to get over the concept that all the rest of the world should adopt the rules we have," Blaschke says. Her prediction: "REACH will be a world regulation. Sooner or later, it will happen, once REACH is up and running."





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter