Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cross-Coupling Made Easy
Iron catalysis simplifies coupling of aryl and alkyl Grignard reagents
by Stephen K. Ritter
January 12, 2009
| A version of this story appeared in
Volume 87, Issue 2
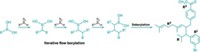
DIRECT CROSS-COUPLING of aryl halides with alkyl halides to form substituted aryl compounds—one of the most indispensable types of reactions in organic synthesis—has just gotten easier thanks to a new procedure that combines the formation of Grignard reagents and iron catalysis in one-pot reactions (Angew. Chem. Int. Ed. 2009, 48, 607).
The approach, designed by Waldemar M. Czaplik, Matthias Mayer, and Axel Jacobi von Wangelin of the University of Cologne, in Germany, builds on previous iron-mediated Grignard cross-coupling methods. But rather than separately preforming large amounts of hazardous organomagnesium reagents, as in standard Grignard coupling reactions, the new sequence forms Grignard reagents in situ. It also avoids the higher cost and toxicity problems associated with palladium and nickel cross-coupling catalysts.
For a typical reaction, the researchers add FeCl3 dissolved in tetrahydrofuran to a flask containing magnesium turnings. They also add tetramethylethylenediamine to serve as a ligand for stabilizing organometallic intermediates. They then add aryl and alkyl bromide reagents and allow the reaction to run for three hours at 0 °C before quenching it and isolating the product.
Iron catalyzes the in situ formation of the Grignard reagents, Jacobi von Wangelin explains. Although the exact details aren't yet known, it appears that alkyliron and arylmagnesium species are at work, he says. As these reagents form, the active cross-coupling catalyst, which the researchers believe is Fe(MgX)2, where X is Cl or Br, also forms.
The team uses a variety of aryl-alkyl and alkenyl-alkyl combinations to make coupling products with up to 81% yields. Slow formation of the Grignard reagents combined with the rapid iron-catalyzed coupling step keeps the concentration of reactive organomagnesium intermediates low throughout the reaction, Jacobi von Wangelin says.
This approach to aryl-alkyl cross-couplings "nicely illustrates a general trend in today's catalytic organic chemistry: to achieve high selectivity with simple, readily available reagents in a safe and environmentally benign manner," notes Carsten Bolm of RWTH Aachen University, in Germany. The Cologne team's reaction is effectively "cross-coupling made easy," Bolm says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter