Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Iodide-Salvaging Enzyme Analyzed Structurally
Crystal structures of iodotyrosine deiodinase, an enzyme required for the efficient recovery and reuse of dietary iodide, have been determined
by Stuart A. Borman
May 25, 2009
| A version of this story appeared in
Volume 87, Issue 21

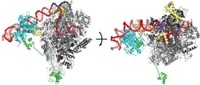
Crystal structures of mammalian iodotyrosine deiodinase (IYD), an enzyme required for the efficient use of dietary iodide, have been determined by a University of Maryland research team (J. Biol. Chem., DOI: 10.1074/
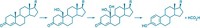
jbc.M109.013458). The work provides a deeper molecular-level understanding of how the enzyme functions and how IYD mutations can cause disease. Iodide is essential for producing the thyroid hormone thyroxine, which helps control metabolism, growth, and development. Insufficient dietary iodide intake, or IYD mutations that make the enzyme unable to recycle iodide, can cause hypothyroidism, a condition that can result in mental impairment. To conserve iodide, IYD works in conjunction with the cofactor flavin mononucleotide (FMN) to recover and reuse iodide from by-products of thyroxine biosynthesis. Nicole LaRonde-LeBlanc, Steven E. Rokita, and coworkers have now obtained the first crystal structures of the dimeric enzyme with and without its substrates, iodotyrosine and diiodotyrosine. The structures reveal that known disease-causing mutations are located in regions that surround the enzyme-bound FMN cofactor. They also reveal structural features that explain how the enzyme selects for its tyrosine-based substrates and avoids removing iodide from thyroxine.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter