Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
New Structure Revisits History
Enzyme played key role in World War I and history of enzymology
by Stu Borman
May 25, 2009
| A version of this story appeared in
Volume 87, Issue 21

AN EXAMPLE OF the way enzymes accelerate reactions, which students have been reading about in textbooks for decades, is wrong and will have to be corrected, according to researchers who have obtained the first detailed glimpse of the structure of acetoacetate decarboxylase (AADase), an enzyme that also played an important role in World War I.
AADase catalyzes the conversion of acetoacetate to acetone. It is a key enzyme in a bacterium that chemist and later Israeli president Chaim Weizmann used in a large-scale process he helped develop to make acetone from starch for the production of explosives during World War I.
The late chemist Frank H. Westheimer used AADase as a prototype for the microenvironment effect—the notion that steric, electronic, and other interactions among enzyme active-site residues profoundly influence the way those enzymes do their jobs. This idea seems commonplace today, but Westheimer and others pioneered it in the 1960s, in work that gave the then-nascent field of mechanistic enzymology a firm foundation.
Westheimer showed that AADase's lysine-115 was able to speed the decarboxylation of acetoacetate to acetone because it was much more acidic than lysine normally is. He speculated that this was due to a "proximal positive-charge effect" from the ammonium group of a neighbor, Lys-116.
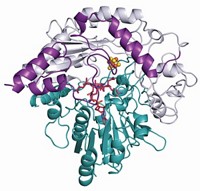
Now, Karen N. Allen of Boston University and coworkers have obtained the first structure of AADase (Nature 2009, 459, 393). It shows that Lys-116 is facing away from the active site and thus can't exert any substantial influence on Lys-115.
The researchers find that the catalytic residue is instead unusually acidic because it is located in a "hydrophobic funnel" structure. This type of protein fold has not been observed previously, and in the paper Allen and coworkers suggest naming it the "Westheimer fold."
This hydrophobic environment makes the positive charge on lysine's terminal ammonium group chemically uncomfortable, giving the amino acid a greater than normal incentive to release a proton and thus become neutral. This confirms rather than detracts from Westheimer's concept of a microenvironment effect, albeit in a different way than Westheimer envisioned.
"Although never directly demonstrated," Westheimer's proposal "became and remained a textbook teaching point," comments enzymologist Thomas K. Harris of the University of Miami. "The structural findings create new excitement, in the sense that they reopen the door for enzymologists to discover and characterize an enzyme to replace AADase as the protypical example of the proximal positive-charge effect."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter