Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
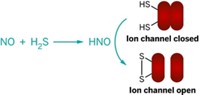
HNO Generates Unique Chemical Markers
Nitroxyl reacts with organic phosphines, yielding unique amide products that can be used to detect the biologically active nitrogen oxide
by Stuart A. Borman
June 15, 2009
| A version of this story appeared in
Volume 87, Issue 24
The first reaction of nitroxyl (HNO) with organic phosphines has been achieved, yielding unique HNO-based amide products that can be used to detect HNO in biological systems (Org. Lett., DOI: 10.1021/ol900914s). HNO, the reduced protonated form of the biological signaling agent nitric oxide, promotes heart contractions and is therefore considered to be the basis for potential congestive heart failure medications. But "the lack of specific HNO detection methods has impeded the understanding of HNO in biological systems," write S. Bruce King and coworkers of Wake Forest University. They report that HNO reacts with triarylphosphines to produce reactive aza-ylides, which then react immediately with a neighboring ester group to generate stable and unique amide products. The amides can thus be used as markers for HNO in biological systems. According to the researchers, these experiments document the reactivity of HNO with triarylphosphines and provide insight into a new method for distinguishing this important biologically active nitrogen oxide.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter