Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Academic Award
by Stephen K. Ritter
June 29, 2009
| A version of this story appeared in
Volume 87, Issue 26

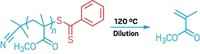
Carnegie Mellon University chemistry professor Krzysztof Matyjaszewski received the Academic Award for developing atom-transfer radical polymerization (ATRP) techniques that use small amounts of a copper catalyst in conjunction with environmentally friendly reducing agents or radical initiators. The technology has opened up greener routes to advanced polymeric materials.
Polymers prepared by radical polymerization were initially limited to making commodity plastics, rubbers, and fibers because of a lack of structural control. But when Matyjaszewski and colleagues discovered ATRP in 1995, it made production of functional polymers with precisely controlled molecular architectures possible. These polymers are being used as specialty coatings and recyclable elastomers in the automotive industry, optoelectronic applications, and pharmaceutical and cosmetic areas.
In ATRP, a Cu(I)-based catalyst, or "activator," is continually oxidized to a Cu(II) species during polymerization and replenished by recycling. Because of unavoidable radical termination reactions, replenishment is incomplete. Thus the polymerization requires high concentrations of copper, some of which ends up in the polymer. Since 2004, Matyjaszewski's group has been optimizing the chemistry by incorporating new ligands, reducing agents, or radical initiators. These adjustments have improved the efficiency of regenerating the Cu(I) catalyst, dramatically decreasing the amount of copper needed.
The result is several novel processes: activators generated by electron transfer (AGET), activators regenerated by electron transfer (ARGET), and initiators for continuous activator regeneration (ICAR). ARGET, for example, reduces the amount of copper catalyst from more than 1,000 ppm to around 1 ppm by using amine, sugar, or ascorbic acid reducing agents. And in ICAR an organic radical initiator such as AIBN (azobisisobutyronitrile) serves the same purpose.
Worldwide production of synthetic polymers is 400 billion lb per year, about half of which involves free-radical polymerization, Matyjaszewski notes. "Often hazardous chemicals are used to produce these important industrial products," he says. "We've been able to use environmentally friendly chemicals, such as vitamin C, to reduce the level of catalyst employed in ATRP by a factor of more than 1,000, which both enhances the scope of the procedure and reduces the environmental impact of polymer fabrication."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter