Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Frustrated Couple Settles For Gases
Lewis acid-base pair held apart by their substituents finds a way to reversibly trap N2O and CO2 for possible storage or reaction chemistry
by Stephen K. Ritter
July 13, 2009
| A version of this story appeared in
Volume 87, Issue 28

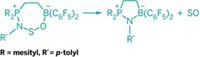
A good love story is hard to come by in chemistry, so when one does come along, it's worth noting. That's the case for the Lewis acid-base pair B(C6F5)3 and P[C(CH3)3]3. The electron-deficient boron would dearly like to accept phosphorus' lone pair of electrons, and phosphorus would freely give the electrons to boron, if the bulky perfluorophenyl and tert-butyl groups weren't standing in the way. Some good has already come from this forbidden affair: The so-called frustrated Lewis pair can serve as the basis of a metal-free catalyst. In a new twist to the plot, Douglas W. Stephan of the University of Toronto and coworkers report that exposing a solution of B(C6F5)3 and P[C(CH3)3]3 to N2O leads to insertion of the gas between the phosphorus and boron. Phosphorus forgets about boron and donates its lone pair to a nitrogen atom, and the jilted boron takes an electron pair from oxygen (J. Am. Chem. Soc., DOI: 10.1021/ja904377v). Separately, Stephan's group teamed up with Gerhard Erker, Stefan Grimme, and coworkers of the University of Münster, in Germany, to use CO2 as the third party (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200901636). The reversible trapping of N2O and CO2 could provide a means of capturing the greenhouse gases for storage or for activating the molecules for chemical reactions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter