Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Smaller Is Better
Subnanometer catalyst particles are unexpectedly active
by Mitch Jacoby
July 27, 2009
| A version of this story appeared in
Volume 87, Issue 30

A dendrimer-based method can controllably synthesize 1-nm- and subnanometer-sized metal particles containing a well-defined number of atoms, according to researchers in Japan. The team describes an unprecedented solution-phase route to particles in that size range and reports that they are unexpectedly active catalytically (Nat. Chem., DOI: 10.1038/nchem.288).
Given that particles of 3-nm diameter are more active catalytically than their 6-nm counterparts, it might stand to reason that 1- or 0.5-nm particles would be better still. Yet conventional wisdom and some experiments say differently. For the oxygen-reduction reaction (ORR), for example, a reaction that occurs in common fuel cells, platinum particles measuring approximately 3 nm are regarded as the best catalysts.
Now, Kimihisa Yamamoto and Takane Imaoka of Keio University and coworkers report that platinum’s ORR catalytic activity continues to increase with decreasing size as the particles are shrunk to less than 1 nm.
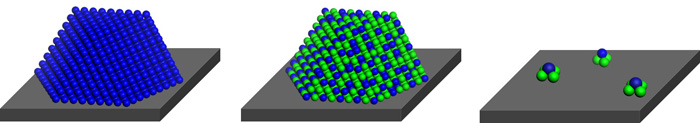
To make the particles, which fall in a size range that has been difficult to access controllably, the team designed a novel dendrimer based on phenylazomethine units that form one-to-one complexes with platinum salts. By treating separate aliquots of the dendrimer solution with a selected number of equivalents of platinum chloride in a stepwise fashion, the team produced dendrimers with increasing numbers of platinum atoms. When they reduced the products, the platinum atoms coalesced into size-selected clusters within the dendrimers.
On the basis of microscopy analysis, the team says the method yielded clusters (or particles) measuring 0.9, 1.0, and 1.2 nm, consisting of 12, 28, and 60 atoms, respectively. ORR catalysis tests comparing electrodes modified with the dendrimer-caged clusters show that the smallest cluster is the most active. In addition, the 0.9-nm catalyst is 13 times more active than a commercial 2.5-nm platinum catalyst, which “goes against current thinking,” Yamamoto says.
“While it is clear that dendrimer templating has much to offer from a synthetic perspective, quite a lot of work remains to be done before a complete understanding of the relationship between nanoparticle size and electrocatalytic activity will emerge,” notes Richard M. Crooks, a chemistry professor at the University of Texas, Austin. This study represents a thought-provoking step in that direction, he adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter