Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Prioritizing Chemicals
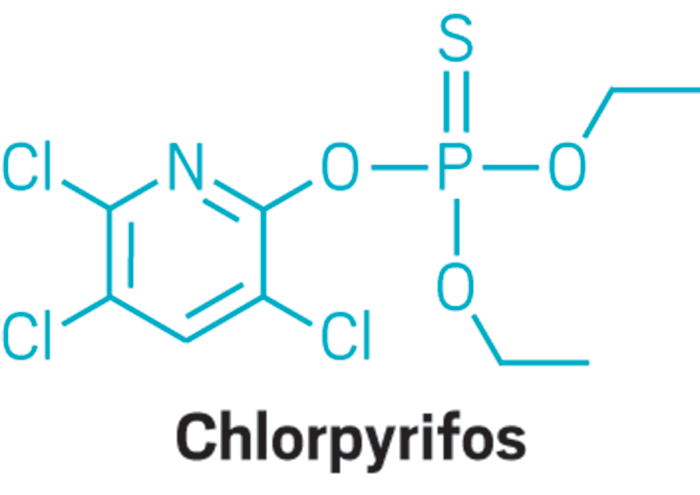
EPA considers hazard, not just exposure, for future screening of endocrine disrupters
by Britt E. Erickson
September 14, 2009
| A version of this story appeared in
Volume 87, Issue 37

To help identify chemicals for future screening under its Endocrine Disrupter Screening Program (EDSP), the Environmental Protection Agency is considering prioritization approaches that look beyond exposure and consider effects or the likelihood that a chemical is an endocrine disrupter. The goal is to limit use of costly, time-intensive in vivo animal testing to chemicals that pose the greatest hazard.
EPA selected the first batch of chemicals that will undergo screening, not because they are suspected of being endocrine disrupters, but because of their high potential for human exposure through food and water. Now the agency is under pressure from Congress to expand the program beyond that initial list of 67 pesticide chemicals, which was announced last April (C&EN, April 20, page 32). As a result, EPA is busily trying to determine how it will select the next batch of chemicals for screening.
EDSP meets a mandate under the Food Quality Protection Act of 1996 for screening of all pesticide chemicals—both active and inert ingredients—for potential estrogenic effects in humans. EPA can require testing of chemicals other than pesticides if a substantial human population is exposed to them. It also has the authority to order tests for androgen or thyroid effects, as well as endocrine effects in species other than humans.
Because of the vast number of chemicals that may be endocrine disrupters, EPA is considering a combination of computational and high-throughput in vitro methods to prioritize which compounds to screen next. Computational methods involve quantitative structure-activity relationships (QSARs), which predict a chemical’s behavior on the basis of structure and other physicochemical properties through sophisticated computer models.
EPA did consider such approaches early in the development of EDSP, said Gary Timm, a scientist with EPA’s Office of Science Coordination & Policy, during a meeting of the Federal Insecticide, Fungicide & Rodenticide Act Science Advisory Panel (SAP) on Aug. 25. But back then, the high-throughput assays “did miserably,” he said, and QSARs “didn’t perform much better.”
When asked to elaborate on the failure of high-throughput screening, Timm replied that when EPA tested 61 well-studied chemicals with such assays in the late 1990s, the results were “not even close” in terms of predicting known effects. The problem, he said, was that the assays were designed by the pharmaceutical industry for use with potent drugs, not with substances having weak estrogenic activity.
EPA decided then that high-throughput assays had to be optimized before they could be used in EDSP. The agency set them aside because of resource constraints and proceeded to validate other, more traditional assays, Timm said. That process has taken more than a decade and is still incomplete for some of the more extensive, multigenerational in vivo assays.
EPA is now taking a second look at high-throughput screening assays and computational methods for prioritization purposes. This reconsideration is especially important for chemicals for which there is minimal toxicological data, Steven Bradbury, deputy director in EPA’s Office of Pesticide Programs, told SAP in August.
Those chemicals include inert pesticide ingredients and non-food-use active pesticides. Inert pesticide ingredients such as solvents, emulsifiers, carriers, thickeners, and pH control agents are especially challenging because they have many different types of chemical structures and vary widely in physicochemical properties, Bradbury noted. More than 1,000 inert ingredients have food-use approvals, he added.
Non-food-use active pesticides, also called antimicrobials, include sanitizers, disinfectants, slimicides, antifoulants, and preservatives. About 50% of these chemicals are registered for public health use. There are more than 250 registered antimicrobials and more than 5,000 registered products that contain antimicrobials, Bradbury said.
Focusing on these two classes of chemicals, researchers at EPA’s Office of Research & Development (ORD) and its Office of Pesticide Programs have developed a QSAR-rule-based expert modeling system that predicts a chemical’s binding affinity to the estrogen receptor on the basis of the compound’s physicochemical properties, such as whether it is acyclic or has a low octanol-water partition coefficient, which is a measure of hydrophobicity.
The system was built on the hypothesis that “only a small percentage of these chemicals are likely to bind to the estrogen receptor,” according to Patricia K. Schmieder, a researcher at EPA’s ORD/National Health & Environmental Effects Research Laboratory, in Duluth, Minn. Energy and steric constraints dictated by the estrogen receptor determine what chemical structures can bind to it, she pointed out at the SAP meeting.
“Inert ingredients and antimicrobial pesticides are nonsteroidal and do not contain multiple hydrogen-bonding groups at the distance needed for steroidlike interactions,” Schmieder noted. Thus, any inert pesticide ingredient or antimicrobial that binds to the estrogen receptor is likely to do so “through an interaction mechanism that results in low-affinity binding.”
The expert modeling system conforms to principles for QSAR validation as outlined by the Organization for Economic Cooperation & Development (OECD). Those principles require that the regulatory purpose of a QSAR be well defined. For EDSP, QSARs would be used to set priorities to support screening decisions and not to make final assessments, Bradbury noted.
OECD principles also require a well-defined end point. In this case, the end point is binding to the estrogen receptor. EPA officials at the meeting emphasized that just because a chemical binds to the estrogen receptor doesn’t necessarily mean that the chemical is an endocrine disrupter. The agency intends to use binding-affinity data to prioritize which chemicals should be further screened under EDSP.
Representatives from the pesticide trade group CropLife America and the Biocides Panel of the American Chemistry Council (ACC), a chemical industry trade group, expressed concerns about the expert system in comments they submitted to SAP.
In particular, ACC’s Biocides Panel noted that the scope of the system is inadequate. “The proposed expert system will not be sufficiently broad to address the inventories of interest,” the panel commented. “The scope of antimicrobial active ingredient chemicals apparently not covered by this expert system is very extensive.” ACC is concerned that chemicals that can’t be or have not been assessed by the system will be considered higher priority simply because the sorting system cannot address them.
CropLife America’s concern is more about the “big picture.” In comments submitted to SAP, Erik R. Janus, director of human health policy at CropLife America, questioned why EPA is focusing its expert system on chemicals that are unlikely to be endocrine disrupters.
“According to past work, only 4% of inerts and 7% of antimicrobials showed ‘higher probability’ of estrogen binding affinity. Is there merit in identifying more ‘negatives’?” he asked.
Both groups also claimed that the amount of time SAP and the public had to review the expert modeling system was insufficient. They recommended greater transparency and communication with stakeholders.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter