Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Promoter Quadruplexes
Folded DNA structures in gene-activation sites may be useful cancer drug targets
by Stu Borman
November 2, 2009
| A version of this story appeared in
Volume 87, Issue 44

Designers of cancer drugs have begun to aim their arrows at an up-and-coming series of targets in the human genome: quadruplexes, a family of DNA structures that form in sites that switch genes on and off.
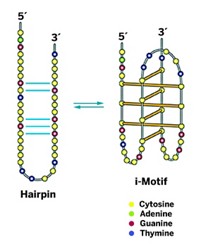
Quadruplexes form when sets of guanine nucleotides in guanine-rich DNA sequences hydrogen-bond to form flat “tetrads” that then stack up like floors in a molecular building. They form transiently in genomic DNA, notably in gene switches, also called promoters, where they help orchestrate the symphony of gene expression in living cells.
Gene promoters where quadruplexes form include those of oncogenes, which are genes that initiate cancers and help them grow. Quadruplexes can also be induced to form in the end caps of chromosomes, called telomeres, which are also implicated in cancer. That’s why quadruplexes are considered promising targets for antitumor medications.
A better understanding of how quadruplexes work has begun to emerge. It turns out that these folded DNA structures are generally obstructionists at heart. In promoters, they appear to tie up DNA, making it difficult for transcription-factor proteins to bind to promoter sites and do their job of turning gene transcription on or off. They may also obstruct telomeres, making it difficult for the enzyme telomerase to bind and catalyze telomere extension. These inhibitory actions can shepherd cancer cells to a hasty demise.
In recent years, quadruplexes have been characterized in oncogenes such as c-myc, bcl-2, and c-kit. The findings were questioned, with some researchers noting that quadruplexes can form only in single-stranded DNA, whereas most genomic DNA, including promoter DNA, is double-stranded.
“Biologists have been most skeptical,” says quadruplex specialist Laurence H. Hurley of the University of Arizona, Tucson. “They’ve asked, ‘How can you get from duplex DNA to a single-stranded element that can form these structures?’ ” Quadruplexes are also more thermodynamically stable than duplex DNA, Hurley notes, and some skeptics have also questioned how cells get rid of quadruplexes once they form, if they do indeed form.
Recent studies have addressed these questions. As to how quadruplexes can form, senior investigator David L. Levens of the National Cancer Institute and coworkers have found that when a gene threads itself through RNA polymerase during transcription, it causes underwinding of DNA behind the polymerase, where the promoters are found (Nat. Struct. Mol. Biol. 2008, 15, 146). Underwinding induces duplex DNA to open into single strands.
A quadruplex can form in one of these single strands, and another type of folded DNA structure called an i-motif can form in the other strand. The quadruplex and i-motif may then prevent gene-activating transcription factors from binding to the promoter. The blockage would turn off gene transcription. Such open regions of DNA may also recruit other factors that modulate transcription.
Regarding how cells get rid of quadruplexes they no longer need, Hurley’s group and that of Shantanu Chowdhury of the Institute of Genomics & Integrative Biology, in New Delhi, have identified helper proteins that facilitate the formation or unfolding of quadruplexes. For example, the protein nucleolin binds and stabilizes gene-promoter quadruplexes, ensuring that the transcription switch for the corresponding gene is in the off position. Other helper proteins promote quadruplex breakdown.
Such models of how quadruplexes work in promoters could aid drug design. For instance, about 80% of cancer cells overexpress c-myc protein, which normally would make it a good drug target. “The problem is that the protein itself has been an undruggable target,” Hurley says. Not only does it exist only fleetingly, but an inhibitor of c-myc protein “would probably have to block a protein-protein interaction, which is very difficult,” he says.
Turning off transcription of the c-myc gene by stabilizing the c-myc promoter quadruplex with a small molecule may be a good alternative to targeting the protein itself. In 2002, Hurley’s group demonstrated that the small molecule TMPyP4 could interact with the c-myc quadruplex and turn off transcription.
In 2007, pharmaceutical scientists Zhi-Shu Huang and Lian-Quan Gu of Sun Yat-sen University, in Guangzhou, China, and coworkers found that quindoline derivatives could cause c-myc quadruplexes to form and also stabilize the structures, causing downregulation of c-myc expression.
And this year, Hyun-Jin Kang and Hyun-Ju Park of the College of Pharmacy at Sungkyunkwan University, in Suwon, South Korea, found that the antibiotic actinomycin D likewise binds to the c-myc promoter quadruplex and inhibits transcription. They are trying to develop analogs as potential anticancer agents.
Quadruplexes may also be useful alternative targets when resistance renders a protein-targeted cancer drug useless. For example, “kinase enzymes are druggable targets but tend to become drug resistant,” says University of London’s Stephen Neidle, a specialist in drug-nucleic acid interactions. Mutations in the site where kinase inhibitors bind cause the resistance.
“Many oncologists say the resistance problem is the single biggest challenge to effective therapy in many cancers and really is clinically an enormous problem,” Neidle says. Targeting a kinase promoter quadruplex could be a useful workaround, he notes.
For example, mutation of the gene for the kinase enzyme c-kit causes gastrointestinal stromal tumors. In current therapy for such tumors, drugs are used to inhibit c-kit protein, but many patients develop resistance to such drugs. This year, Neidle and coworkers described an alternative approach, using a small-molecule naphthalene diimide derivative to stabilize a c-kit promoter quadruplex and thus downregulate c-kit expression. The researchers are now testing this approach in patient-derived cells, including drug-resistant ones.
“A number of labs have looked at promoter regions in different genes,” Hurley says, “and a common theme is emerging: Quadruplexes form there, and you can modulate transcription by targeting them with small molecules.”
Cancer cells depend on the expression of oncogenes like c-myc, whereas normal cells do not. So turning off c-myc expression by targeting oncogene quadruplexes could kill cancer cells without causing irreversible changes that lead to damaging side effects in normal cells.
Whether scientists can design quadruplex-targeted agents that take aim at specific oncogene quadruplexes in the genome remains uncertain.
“Most quadruplex ligands described so far have a limited preference for a given quadruplex topology,” says quadruplex researcher Jean-Louis Mergny of the Inserm U869 Laboratory at Université Victor Segalen Bordeaux 2, in France. “So improving selectivity is a major goal for further studies.”
Nevertheless, because different quadruplexes fold in unique and complex ways, he and others in the field hope quadruplex-targeted drugs can eventually be designed to be as selective as conventional drugs that interact with proteins.
Different promoter quadruplexes adopt different structures, according to medicinal chemistry professor Shankar Balasubramanian of Cambridge University. “Thus, there is ample scope for selectivity in molecular recognition by small organic molecules,” he says. “In considering quadruplexes as therapeutic targets, there is reason to anticipate that we will be able to achieve useful windows of selectivity going forward.”
In any case, investigating quadruplex-targeted drugs is still in its early stages. “We’re still some way away from actual therapeutic molecules being used in the clinic,” Neidle says. “But the amount of evidence from a number of directions is so great that hitting quadruplex targets seems to make sense. With a dozen or more successful studies now in hand, it starts to look as if this could be an important therapeutic approach.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter