Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemical Safety: Unstable Dibromo Intermediate
November 23, 2009
| A version of this story appeared in
Volume 87, Issue 47
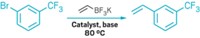
Chemists at Merck & Co. were carrying out a published procedure for preparing 2-bromo-3-methylbutenal (Org. Lett. 2007, 9, 4607). The reaction and workup were successfully carried out twice on a 10-g scale and then scaled up to prepare 600 g in 8 L of dimethyl sulfoxide (DMSO). While large-scale reaction mixture was being heated to 45 °C, however, the temperature unexpectedly and very rapidly rose to well over 100 °C, and material boiled out of the condenser. This exotherm subsequently occurred in a gram-scale run as well.
When the Environmental & Process Safety Engineering group at Merck evaluated this procedure, several potential hazards were identified. The reaction mixture has a secondary decomposition with an onset near 100 °C. The isolated dibromo intermediate compound is thermally unstable and rapidly degrades on standing to a red-black tarry substance.
The dehydrobromination rate of reaction was found to be a strong function of the amount of time the dibromo intermediate was held before charging to DMSO, with the reaction becoming more rapid as the intermediate ages. Finally, attempts to determine the source of the secondary decomposition, which produces a large amount of gas, showed both the hydrobromic acid and the product haloacrylate produced in the dehydrobromination reaction lowered the stability of DMSO.
We wanted to alert the chemical process industry to the risks associated with this particular procedure. Anyone contemplating use of this chemistry should thoroughly evaluate its safety.
Tom Vickery, Ephraim Bassan, Ronsar Eid, Erik Dienemann, Derek Von Langen
Merck & Co., Rahway, N.J.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter