Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Calcite Close-Up
Composites: 3-D images reveal mineral’s interactions with a biopolymer
by Bethany Halford
November 30, 2009
| A version of this story appeared in
Volume 87, Issue 48
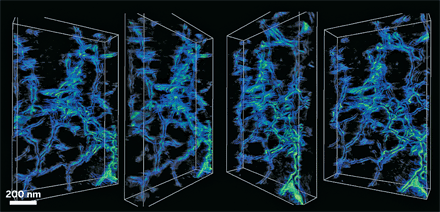
Using electron tomography, scientists have gotten a glimpse inside calcite crystals laced with the polysaccharide agarose (Science 2009, 326, 1244). Their three-dimensional images reveal nanoscale interactions between the mineral and the biopolymer, which could provide clues about how similar composites behave in biological systems.
A menagerie of underwater creatures use biopolymers to orchestrate the formation of calcite, a polymorph of CaCO3. The single-crystal calcite spines of sea urchins incorporate tissue and proteins, and the calcite prisms that make up mollusk shells contain proteins and other biomacromolecules. These biogenic calcite crystals possess unusual physical properties, such as enhanced strength and toughness. Until now, no one had observed the interaction between calcite and an incorporated biopolymer up close.
Cornell University’s Hanying Li, Huolin L. Xin, David A. Muller, and Lara A. Estroff reasoned they could peer deeply into the calcite matrix with a combination of annular dark-field scanning transmission electron microscopy and annular dark-field electron tomography. So they grew calcite single crystals that contain high amounts of agarose and used their instruments to take extreme close-ups.
“Being able to image the organic aggregates in the calcite and see how the crystal incorporates them will help us design synthetic systems that are similar to the biological ones,” says Estroff, the study’s lead researcher.
The calcite single crystals incorporate agarose as a 3-D network of nanofibers, rather than as isolated molecules or individual fibers, the researchers found. At high magnification, they also observed that the calcite crystalline matrix accommodates the fibers’ curvature with facets where the organic and inorganic materials meet. The results suggest that the biopolymers become physically entrapped in calcite crystals, the researchers say, and that no specific chemical interaction drives their incorporation.
The upshot of that conclusion is that all manner of organic molecules can be incorporated via this mechanism. This is likely to be welcome news to those trying to design synthetic biocomposites.
“It is now clear that crystals aren’t the places where molecules and ions go to die; rather, they have extraordinarily rich lives when considered from the point of view of micromolar and submicromolar inclusions,” comments Bart Kahr, an expert in crystalline materials at New York University. “While it is clear that crystals can serve as hosts to all manner of strange guests, descriptions of the local interactions between crystalline matrices and inclusions are few and far between. Estroff and coworkers take this analysis to new heights with the latest techniques in electron microscopy.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter