Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalytic Simplification
Nonenzymatic technique for enantioselective amine acylation dispenses with a complex catalyst
by Stuart A. Borman
November 30, 2009
| A version of this story appeared in
Volume 87, Issue 48

The ability to acylate amines enantioselectively by using a small-molecule catalyst instead of an enzyme just got a bit easier. A Rutgers University group has made this challenging task more convenient by eliminating the need to use an expensive chiral nucleophilic catalyst to carry it out.
Enantioselective catalytic acylation is used in organic synthesis to acylate amines and in drug discovery and basic research to isolate enantiomers by kinetic resolution of racemic amines. But until almost a decade ago, the nitrogen atom of racemic amines could be acylated enantioselectively only by using enzymes. Chemists would like to be able to control the process more directly by mimicking enzyme action with small-molecule catalysts.
In 2001, Gregory C. Fu and coworkers at Massachusetts Institute of Technology succeeded in doing that for the first time (Angew. Chem. Int. Ed. 2001, 40, 234), using an O-acylated azlactone as an acylating reagent and a chiral 4-(dimethylamino)pyridine (DMAP) derivative as a nucleophilic catalyst. Together they form an ion pair that acylates racemic primary amines with selectivity factors up to 27—the fast-reacting enantiomer is acylated 27 times more rapidly than the slow-reacting one.
But, the azlactone is structurally complex and the chiral catalyst is expensive—factors that have discouraged widespread adoption of the approach.
Daniel Seidel and coworkers at Rutgers have now developed a catalytic asymmetric amine-acylation method that uses simpler reagents and dispenses with the chiral nucleophilic catalyst (J. Am. Chem. Soc., DOI: 10.1021/ja9079435).
In their technique, benzoic anhydride—a cheap, commercially available compound—and a chiral thiourea are mixed with DMAP, an achiral nucleophilic catalyst. Together they form an ionic acylating agent that acylates amines enantioselectively with selectivity factors up to 24—comparable with selectivities of Fu's technique.
Seidel and coworkers demonstrated the capabilities of the technique by using it to acylate racemic amines and then resolve them into enantiomers. "We anticipate that the new concept will find applications in numerous other reactions," Seidel says.
Vladimir Birman of Washington University in St. Louis, who specializes in asymmetric catalyst design and nonenzymatic kinetic resolution, comments that "the work introduces a conceptually new approach for nonenzymatic enantioselective acylation of amines." Not only can it be used for kinetic resolution of amines but also potentially for related techniques, such as dynamic kinetic resolution of amines and desymmetrization of meso-diamines. In addition, the approach may prove applicable to enantioselective acylations of other classes of substrates besides amines, Birman notes.

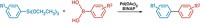

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter