Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Fine Chemicals Firms Continue To Expand
At Informex, companies touted investments in facilities and technology
by Ann M. Thayer, C&EN Houston, and Rick Mullin, C&EN Northeast News Bureau
February 16, 2009
| A version of this story appeared in
Volume 87, Issue 7

ALTHOUGH MORE SUBDUED than in recent years, fine and custom chemical manufacturers at the annual Informex trade show in San Francisco last month continued to talk expansion. There's no doubt that the global economic crisis is dragging on business, but suppliers are still adding production and technology capabilities, if only to stay competitive.
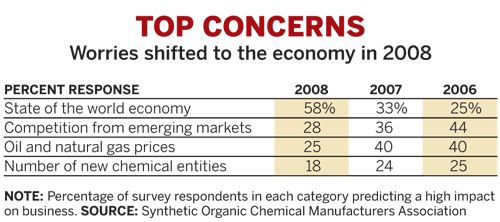
More than 70% of the nearly 100 Synthetic Organic Chemical Manufacturers Association members who responded to the trade group's annual survey in late 2008 still see market conditions as good to excellent, SOCMA reported at the meeting. This optimism, however, has dimmed from 2007 when more than 92% of respondents indicated that they were upbeat.
Industry conditions also have been weakening, with 27% of surveyed firms reporting a sales decline in 2008, compared with only about 18% in 2007. And SOCMA expects the downward trend to continue through this year.
Despite the gloomy outlook, companies told SOCMA that they intend to invest in R&D this year at levels comparable with those of the previous two years. Likewise, capital spending remains steady at about 10.5% of sales. In 2009, new product offerings, broader technology capabilities, and process improvements still are viewed as the means to boosting profits.
Among the industry's larger investments, Cambrex is starting up a $20 million expansion of its active pharmaceutical ingredient (API) plant in Karlskoga, Sweden, this spring. The company has added 8,500 L of capacity in two new processing lines. It is also opening a new current Good Manufacturing Practices (cGMP)-compliant purification plant in Milan, Italy. The $30 million investment includes a warehouse with automated conveyors and bar code scanning. The Milan complex is dedicated to making APIs for generic drugs.
Eric P. Neuffer, vice president of sales and business development for the company's pharmaceuticals business in North America, said Cambrex has had no second thoughts about investing during a worldwide recession. "We believe that it was required for our facilities to stay competitive," he said. "We believe we can keep it full."
SALTIGO, the custom chemical arm of Lanxess, has invested approximately $10 million to upgrade API production in Leverkusen, Germany, and Redmond, Wash., and is teasing out incremental expansions at all of its facilities, according to Andreas Stolle, head of pharmaceutical chemicals. He characterized business as stable, noting that large drug companies are trending toward increased outsourcing.
On the other hand, Uwe Brunk, Saltigo's head of agricultural and specialty chemicals, said the agchem business has never been better than it was last year. The effect of the recession on Asia and other growing economies could affect business this year, however. "We are cautiously optimistic based on current sales of seeds and fertilizer," Brunk said. "March will be very important—we will see if farmers are buying pesticides."
SAFC, the fine chemicals arm of Sigma-Aldrich, is spending $12 million to expand its new biologics facility in Carlsbad, Calif. Overall, it will spend about $50 million between 2008 and 2009, much of it on capacity to synthesize highly potent compounds, according to SAFC President Gilles A. Cottier. Similarly, Carbogen Amcis will open a $25 million high-potency facility in Balva, India, by midyear.
Meanwhile, Novasep has invested $55 million over the past year in high-potency API production at its plant in Le Mans, France, and at a new biologics facility in Pompey, France. Almac Sciences, Ash Stevens, and Excelsyn were among other Western firms announcing smaller scale expansions at Informex.
A year or two ago, the fine chemicals industry was most concerned about energy prices and competition from emerging markets, according to the SOCMA survey. The focus has since shifted to the economy, but suppliers still expect that competitors from emerging regions will gain ground. Such firms are predicted to hold more than 30% of the market in 2009.
It's not surprising, then, that many plans presented at Informex related to expansions in India and China. Such announcements often came from companies stressing that they couple a U.S. base—from which they'll handle logistics and contacts with Western customers—with low-cost manufacturing operations overseas.
This combination is intended in part to address growing customer concerns about product quality, particularly from China. As before, 72% of responders to SOCMA's latest survey believe products from emerging market suppliers are of inferior quality compared with those from Western firms. Some think sufficiently good products sometimes come from these firms, but virtually no one believes the products are better.
In fact, SAFC has seen some customers move parts of their supply chains back to the U.S., Cottier said. "India and China will not disappear, but people will be more prudent than in the past five years." At the same time, SAFC continues to build new operations in Asia.
TO HELP ALLAY any fears, Shanghai-based WuXi AppTec has introduced a new global procurement service to help customers buy reagents from qualified Chinese vendors. Not only will the company help buy materials, but it will also ensure their quality by providing quality-control testing and certificates of analysis, along with repackaging and export services.
The service arose from customer requests, explained Steven M. Hutchins, WuXi's vice president for business development. "We have a strong network of suppliers," he added.
WuXi expanded into the U.S. last year with the acquisition of AppTec Laboratory Services. In China, meanwhile, the company is starting up a new general-purpose plant that will quadruple synthesis capacity at its site in Jinshan. The plant has 18 reactors ranging from 8,000 to 20,000 L in size. WuXi puts its annual capacity for intermediates and APIs at 50–100 metric tons.
Also bridging East and West, Asymchem Laboratories is based in Morrisville, N.C., and manages projects from the U.S., explained Richard W. Fengl, director of business development. Yet it produces pharmaceutical chemicals in several wholly owned facilities in China. Part of its integrated offering is a new program to quickly move drug candidates through development and into Phase I clinical trials.
In March, Asymchem will open the first stage of a cGMP-compliant high-potency API facility in Tianjin. It is converting an existing pilot plant to handle potent compounds in two suites and a formulation module. According to Fengl, the company believes its plant will be the first cGMP-compliant high-potency facility in China.
In a similar first, AmbioPharm claims to have the first cGMP-compliant peptide plant in China, said Jim Hampton, vice president of business development. The company's operations are split between the U.S. and China; it acquired its South Carolina cGMP and process development facility from UCB in 2006. By 2010, it expects to run a dedicated manufacturing line in China for a generic peptide drug.
Pleasanton, Calif.-based Chemlex Pharmaceuticals, which advertises "pharmaceutical ingredients made in China under U.S. management," also intends to expand its Chinese operations substantially. Already employing 30 chemists and engineers, it plans to add 50 hires and put a new 100-metric-ton-per-year plant into production this year. In May, Chemlex will open a new R&D center in Suzhou, China.
India got attention, too. CiVentiChem said it is proceeding with the construction of a $7 million, 20,000-L API plant with R&D facilities in Hyderabad, India. The firm also announced it will spend about $1 million on a 100-L cGMP-certified plant and new analytical services at its headquarters in Cary, N.C.
CiVentiChem President Bhaskar R. Venepalli said the firm is stepping up operations to meet the needs of current customers whose projects are advancing, but also to have the capacity to expand its business. "We are a little concerned with things going on right now," he said. "But I think things will turn around in the months ahead."
IN ANOTHER geographic shift, European companies are strengthening their presence in the U.S. During Informex, Switzerland's Helsinn announced that it had acquired Sapphire Therapeutics, which will give it R&D and marketing support in Bridgewater, N.J.
France's PCAS is launching a joint venture with Nanosyn, a contract research firm in Menlo Park, Calif., that will provide process R&D and kilogram-to-commercial-scale manufacturing for PCAS customers in North America. The firms plan to acquire a production site in the San Francisco area and to be offering about 200 L of cGMP capacity in two or three suites by September, according to Joseph Tessier, vice president of PCAS America.
Companies also continue to invest in innovative processes to differentiate themselves from their competitors and to attract customers. Notable examples at Informex were head-to-head presentations by DSM and SAFC—in front of a standing-room-only audience—on microreactor-based continuous-process technologies that the firms are putting into play commercially.
Other companies, meanwhile, promoted their chiral synthesis technologies, solid-state chemistry capabilities, metal-scavenging materials, and transition-metal and biological catalysts. To appeal to a broad customer base, companies also advertised their ability to span small- and large-scale production, to handle small and large molecules, and to synthesize potent drugs.
Certain abilities are always touted on the banners and brochures that custom chemical manufacturers display at Informex. But now more than ever, financial concerns are taking center stage as they see customers, especially small drug developers, conserving cash and scaling back spending. Thus, along with the usual quality, reliability, and desire to meet customer needs, many suppliers in San Francisco were emphasizing a very basic trait: their own stability as ongoing business concerns.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter