Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making Hydrogen Peroxide Directly
Acid treatment of catalyst support could simplify production of chemical
by Jyllian N. Kemsley
February 23, 2009
| A version of this story appeared in
Volume 87, Issue 8

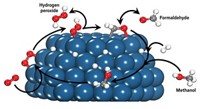
PRETREATING THE SUPPORT for a gold-palladium catalyst used to produce hydrogen peroxide from H2 and O2 could provide a simpler, more direct method than that now used to prepare the commodity chemical, reports a research group led by Graham J. Hutchings, a professor of chemistry at Cardiff University, in Wales (Science 2009, 323, 1037).
H2O2 is used commercially for disinfection and bleaching; the global market for the chemical is expected to reach 3.8 million tons this year, according to a 2008 report by Global Industry Analysts. The current method to produce H2O2 is an indirect process that involves sequential hydrogenation and oxidation of an anthraquinone. Direct methods tend not just to hydrogenate O2 into H2O2 but also to convert H2O2 into water.
Hutchings and colleagues, however, have now found that pretreating an activated carbon support with either nitric or acetic acid, then drying the support before adding gold and palladium, results in a reusable catalyst that produces H2O2 with minimal conversion to H2O.
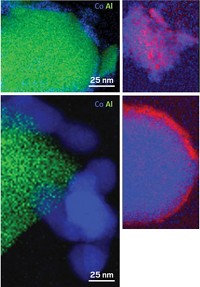
Drawing from a combination of hydrogenation experiments and scanning transmission electron microscopy of prepared catalysts, the researchers propose that acid pretreatment of the catalyst support leads to smaller and better dispersed Au-Pd nanoparticles. The improved nanoparticle dispersion somehow shuts down sites that would convert H2O2 into water, they suggest.
More work needs to be done to evaluate the effect of acid pretreatment for H2O2 synthesis under conditions that more closely mimic a manufacturing setting, Hutchings says.
Nevertheless, "these are intriguing results showing that hydrogen peroxide can be made with high selectivity via a direct process," says D. Wayne Goodman, a chemistry professor at Texas A&M University. "This disclosure will no doubt promote an active search to identify the underlying cause or causes for this spectacular yet poorly understood effect."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter