Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Policy
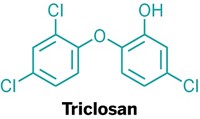
Triclosan Targeted
Regulators question safety, effectiveness of antibacterial in consumer products
by Britt E. Erickson
April 12, 2010
| A version of this story appeared in
Volume 88, Issue 16
Federal regulators are concerned about the potential for antibiotic resistance and endocrine disruption from human exposure to triclosan, an antibacterial ingredient found in numerous consumer products including soaps, body washes, cutting boards, and toys.
"Existing data raise valid concerns about the effects of repetitive daily human exposure" to triclosan, the Food & Drug Administration wrote in a letter to Rep. Edward J. Markey (D-Mass.). The agency was responding to an inquiry from Markey, who sent letters to both FDA and the Environmental Protection Agency in January because of his concern that antibacterial consumer products are ineffective and unsafe for human health and the environment. Markey released both the FDA response and one from EPA on April 8.
In its response, EPA wrote to Markey that "additional research on the potential health consequences of endocrine effects of triclosan is warranted."
FDA regulates triclosan in soaps and hand washes. EPA regulates other products containing the antimicrobial through its pesticides office.
In 2008, EPA reassessed the safety of triclosan, concluding that "human exposure resulting from the use of triclosan in cutting boards, kitchen utensils, toys, and other products did not pose unacceptable risks to human health, including risks to infants and children."
In that assessment, EPA considered data showing that triclosan disrupts thyroid hormone levels in lab animals. But since then, work by EPA researchers has shown that triclosan has potential estrogenic effects in rats. "EPA plans to reexamine the potential risks to human health in light of the new and planned research on the effects of triclosan on the endocrine system," EPA wrote in its letter to Markey.
FDA is in the midst of rulemaking and is considering a petition from environmental groups related to the use of triclosan, the agency noted in its letter. FDA is also working closely with EPA's Endocrine Disruptor Screening Program to assess the endocrine effects of triclosan, the letter said.
In response to FDA's letter, Markey is now calling on regulators to ban triclosan in consumer soaps and hand washes, as well as other products that contact food or are intended for children.
The Soap & Detergent Association, an industry trade group, continues to emphasize the benefits of antibacterial soaps. In a statement issued in response to FDA's concerns, the group claims that soaps containing triclosan are safe and more effective at reducing the risk of bacterial infection than nonantibacterial soaps.
The Natural Resources Defense Council and other environmental groups support a ban on triclosan in personal care products. NRDC is also calling for triclocarban, a similar antibacterial chemical, to be banned from personal care products because of its "widespread use, lack of effectiveness, and concerns for hormone-disrupting effects."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter