Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
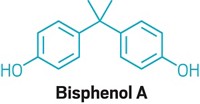
Thanks for Rudy Baum's recent editorial regarding the saga of bisphenol A (BPA) in our current chemophobic society (C&EN, March 1, page 5). I've been very interested in both the public and industrial aspects of this topic because my position in a manufacturing lab has afforded me the opportunity to work with this compound.
In this field, my colleagues and I face exposure to much more serious chemical threats than the ones posed by BPA. We accept our fate every time we walk into a lab. However, recent articles by nonscientists saying this compound poses a new threat like those posed by mercury or lead are unjustified, hasty, and ignorant of all the facts.
I do not condone the indiscriminate use of BPA or any chemical; however, making rash decisions creates a society of poorly educated and fearful consumers. How will our weak economy prosper when the hands of our R&D departments are tied by these fears? For example, thalidomide, a well-documented teratogenic compound, might have found new life as a therapeutic despite its publicly tragic history (C&EN, March 15, page 9).
We should strive to move forward to develop new uses for any compound and remember to take time to fully understand its potential without fear of bad press. I submit that we, the chemical industry and all its proponents, further the education of the general public to the benefits and safe uses of chemicals in our everyday lives. Societies have lived for thousands of years in both fear and awe of chemicals; our society deserves the benefit without the irrational phobia.
Ryan Clark
Fort Mill, S.C.
Baum's editorial on BPA is right on target. I don't understand why BPA has been the singular focus of some endocrinologists and the popular media when all of us are exposed to dozens of other estrogen mimics and antagonists—namely, phytoestrogens. One explanation for this lack of attention is the common but false assumption that synthetic chemicals such as BPA are suspect, but "natural" chemicals are harmless.
These plant-based chemicals include, for example, isoflavones, lignans, and coumestans commonly found in many foods, such as nuts, flaxseed, soybeans, cereals, legumes, beer, and some fruits. Their molecular weights and phenolic structures are typically closer to the structures of human estrogens than is BPA, which also suggests that some phytoestrogens might be more potent estrogen mimics than BPA.
I'm not an expert on phytoestrogens, but it is not hard to find reports of adverse and beneficial effects in animals and humans. For example, red clover reduces the fertility of sheep; coumestrol and genistein (both found in soy) might affect fertility, pituitary function, birth weight, and puberty onset in rats; and a daily diet of soy protein can alter menstrual cycles.
Because the current concern about BPA's potential toxic effects was triggered primarily by controversial low-dose animal experiments, I suggest that existing data and new targeted animal studies on phytoestrogens could help determine how the effects of estrogen mimics, including BPA, in animal models might extrapolate to actual human toxicity—a connection that appears to be vague or missing.
The ubiquitous nature of phytoestrogens also might raise some questions about the control and influence of the diets used in numerous animal studies of BPA, because the metabolic interaction and competition of BPA with dietary phytoestrogens is likely unknown.
Donald Kelsey
Guerneville, Calif.
Should we not point out that BPA is not the epoxy polymer and that the popular press misconstrues the situation? Should we not emphasize more strongly that BPA is not an intentional additive in food containers—that it is, when present, an unintentional by-product/residue of the epoxy polymer (the polymer that provides all of its advantages) and that the BPA itself is present in minute quantities? Perhaps we should offer some numbers.
There is enough misleading information in the popular press, and as chemists, should we not seek to correct the errors and repeat the facts as often as necessary?
Bruce Bernstein
Rockville, Md.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter