Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Selenium’s Role In Mercury’s Toxicity
Structure and binding study shows that Mercury’s “selenophilicity” is greater than its “thiophilicity”
by Stephen K. Ritter
January 11, 2010
| A version of this story appeared in
Volume 88, Issue 2
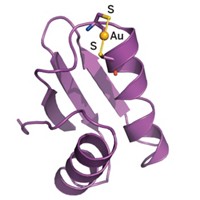
An analysis of how mercury interacts with sulfur, selenium, and tellurium via a protein mimic compound has provided a better understanding of the mechanism of mercury’s toxicity, according to a study by Columbia University’s Gerard Parkin, Jonathan G. Melnick, and Kevin Yurkerwich (J. Am. Chem. Soc., DOI: 10.1021/ja907523x). Mercury toxicity is typically associated with the element’s high affinity for binding sulfur in cysteine residues of proteins and enzymes. A second toxicity mechanism involves mercury’s interaction with selenium, an important antioxidant element in humans. Mercury is known to reduce the bioavailability of selenium by forming insoluble mercury selenide species and by binding to active sites of selenoenzymes. Parkin and coworkers used a tripodal mercaptoimidazolylborate ligand to study mercury’s affinity for sulfur, selenium, and tellurium. One key finding is that the Hg–Se and Hg–Te bonds are shorter than would be predicted from the covalent radii of the elements. The overall X-ray structure evidence coupled with a competitive mercury-binding study involving sulfur and selenium show that mercury’s “selenophilicity” is greater than its “thiophilicity,” Parkin says. Ligands featuring selenium and possibly tellurium may thus prove to be effective in treating mercury poisoning and prompt the design of new chelating agents, Parkin adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter