Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
H-Bonding Enables Molecular Dancing
Transient adsorbed hydrogen facilitates diffusion of organic molecules across a titania surface
by Mitch Jacoby
May 17, 2010
| A version of this story appeared in
Volume 88, Issue 20

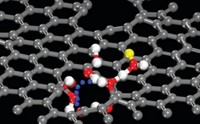
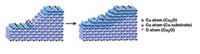
Hydrogen bonding plays a key role in the diffusion of organic molecules across solid surfaces in a dancelike performance, according to a study published in Science (2010, 328, 882). Previous surface studies based on area-averaging techniques have established that hydrogen adsorbed on solids influences diffusion of other adsorbates. But atomic-scale details of such diffusion processes, which can control chemical reactivity and self-assembly on surfaces, have remained largely unknown. With a combination of scanning tunneling microscopy and computational methods, Tulane University’s Shao-Chun Li and Ulrike Diebold (now at Vienna University of Technology, in Austria) and coworkers have worked out a mechanism by which catechol, C6H4(OH)2, moves across a titanium dioxide crystal surface. Upon adsorption, catechol’s OH groups dissociate, leaving the molecule to bind via its oxygen atoms in a bridging configuration to two titanium sites. The liberated hydrogen atoms bind to two surface oxygen atoms, but they can easily shuttle back and forth between TiO2 and catechol. Hydrogen’s shuttling actions make it energetically feasible for catechol to “lift one of its legs” by breaking an O–Ti bond, rotate 180° on the other oxygen leg, rebind to TiO2, and thereby “dance” across the surface via repeated rotations, the team says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter