Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
How Spider Silk Pulls Itself Together
A pair of structural studies shed light on how spiders transform a protein fluid into tough silk fibers
by Carmen Drahl
May 17, 2010
| A version of this story appeared in
Volume 88, Issue 20
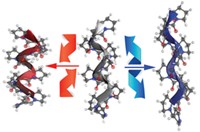
A pair of structural studies have shed new light on how spiders transform a protein fluid into a silk fiber that’s tough as nails. The two independent research teams discovered in lab experiments that spider silk proteins contain molecular switches that respond to changes in ionic composition, flow shear forces, and pH—similar to the changes that occur in a spider’s spinning apparatus. Working with the C-terminal domain of a silk protein from the cross orb-weaver spider, a team led by Horst Kessler of the Technical University of Munich suggests that this domain contributes to the switch between fluid and silk fiber forms in response to changes in salt concentration and mechanical forces (Nature 2010, 465, 239). Meanwhile, researchers led by Stefan D. Knight of the Swedish University of Agricultural Sciences determined that the N-terminal domain of a silk protein from the nursery-web spider sparks polymerization as it experiences a drop in pH (Nature 2010, 465, 236). “The significance of the low pH at the spider’s gland outlet was not clear, and this surprising finding may well provide the answer,” says Uri Gat, a spider silk expert at Hebrew University of Jerusalem, in Israel.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter