Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Catalyst Goes Viral
Nanotechnology: Material grown on a virus template performs better than catalysts grown conventionally
by Bethany Halford
June 14, 2010
| A version of this story appeared in
Volume 88, Issue 24

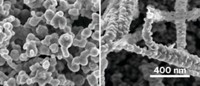
Creating a better catalyst may be as simple as letting a virus do the construction for you. Angela Belcher and coworkers at MIT have used the M13 bacteriophage as a template for growing nanoparticles and nanowires of rhodium and nickel on ceria—a catalyst that can convert ethanol to hydrogen and is therefore promising for fuel cells (ACS Nano, DOI: 10.1021/nn100346h). The virus-templated catalyst possesses significantly different physical properties from catalysts of identical composition grown without the M13 bacteriophage, the researchers report, including improved long-term stability and less vulnerability to surface deactivation.
The M13 bacteriophage can be engineered to assemble nanowires by modifying its surface proteins, which nucleate myriad materials. In the past, Belcher’s group conscripted M13 to make battery electrodes as well as semiconducting and magnetic nanowires.
“The virus is relatively stiff and has a high aspect ratio, so it forms stunning open and porous nanostructures which increase the surface area and shift the pore size distribution,” explains Brian Neltner, the report’s first author. “Surface area and pore size distribution are critical aspects of catalysts, and can improve the reaction rates and selectivity.”
The researchers also found they could eliminate expensive rhodium from the biotemplated material, creating a catalyst with just nickel on ceria. This material can catalytically reform ethanol at temperatures around 400 °C, “offering an alternative, inexpensive catalyst when higher temperatures are acceptable,” they write.
“This new approach to catalyst synthesis clearly provides desirable alternative possibilities for catalytic process applications,” comments Galen Stucky, a chemistry professor at the University of California, Santa Barbara.
“As a materials scientist, it amazes me every day that biology is able to produce a protein which will bind to or nucleate almost any arbitrary material,” Neltner says. “Our lab’s track record of success producing nearly anything we want to make with M13 I think speaks very highly of the incredible versatility of biological systems.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter