Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
FDA Struggles To Keep Up In India
Facility inspections are declining in the country that supplies the most generic drugs to the U.S.
by Jean-François Tremblay
June 14, 2010
| A version of this story appeared in
Volume 88, Issue 24

When Strativa Pharmaceuticals announced in February that it could not launch a new product, the only reason, the New Jersey-based firm said in a statement, was that the U.S. Food & Drug Administration had not inspected the plant in India where the drug was to be made because of “an agency-wide restriction on foreign travel to India.”
A record obtained by C&EN of FDA’s inspections of Indian pharmaceutical facilities during the past several months reveals a sharp drop from December through March, although not a complete halt. The record raises new questions about the consistency and adequacy of the agency’s audits in India.
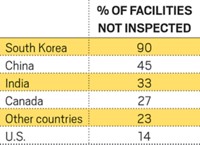
A long-lasting drop in the number of inspections that FDA performs in India would significantly affect the U.S. drug supply. In recent years, India has become the largest foreign supplier of generic drugs to the U.S., with 410 plants producing drug ingredients and drugs in finished-dosage forms.
Without plant inspections, new drugs or cheap generics made in India cannot be approved for sale in the U.S. Moreover, without periodic inspections of existing operations, FDA cannot know whether manufacturers that have been approved are sticking to the high manufacturing standards that initially earned them the agency’s green light.
“There is not now, and there has not been, any recent FDA-imposed nationwide travel restriction to India regarding the FDA’s personnel traveling to or in India,” FDA press officer Rita Chappelle told C&EN in February in response to Strativa’s claim that FDA was not sending inspectors to India. She added that FDA trips were continuing as usual and that India-based FDA staff were free to go to all parts of the country.
Strativa did not respond to C&EN’s request to explain its allegation regarding FDA travel to India. However, when the firm’s parent, Par Pharmaceutical Cos., posted its fourth-quarter results in late February, it said FDA had “lifted” its restrictions on travel to India.
A 2008 report by the Government Accountability Office (GAO), the investigative arm of Congress, brought to light deficiencies in FDA’s foreign inspection program. It concluded that FDA needs to conduct more foreign inspections, keep better records about inspections than it has been doing so far, and promptly conduct follow-up inspections after it uncovers serious violations (C&EN, Nov. 3, 2008, page 17).
David Moore, chief strategy officer at New Jersey-based ChemWerth, tells C&EN that it’s not only plants in India that should be inspected more often. “FDA is woefully underfunded and besieged with applications and requests worldwide.” He adds that foreign manufacturers need a strong U.S. representative to help them avoid unexpected regulatory problems in the U.S. market. ChemWerth is the exclusive agent for numerous Chinese and Indian manufacturers.
But according to Peter Saxon, a New Jersey-based consultant to pharmaceutical manufacturers preparing for FDA inspections, who presently has 17 customers on the subcontinent, “There is definitely something wrong with the FDA’s schedule of inspections in India.”
C&EN obtained FDA’s records of visits in India through a Freedom of Information Act request for the logs of inspections performed by FDA in India for the period from December 2009 to March 2010 and the corresponding year-earlier period. FDA responded with a detailed record of inspections of Indian manufacturing facilities by U.S.-based inspectors during two seven-month periods: from November 2008 to May 2009 and from November 2009 to May 2010.
In addition, the agency handed over its logs of inspections performed by India-based inspectors during 2009 and 2010. FDA opened an office in India in January 2009.
The logs confirm that FDA sharply curtailed its inspections of India in early 2010. From December 2009 to March 2010, U.S.-based manufacturing investigators conducted only five inspections in India compared with 22 one year earlier. During the same period, India-based inspectors visited nine plants, compared with four one year earlier. Overall, the agency conducted 14 inspections in India in the four-month period, compared with 26 one year earlier.
Over the two seven-month periods for which FDA provided data, however, the drop in inspections was not as sharp. Between November 2009 and May 2010, U.S.-based FDA inspectors conducted 31 audits in India, compared with 44 one year earlier. Meanwhile, India-based inspectors conducted 10 inspections compared with four in the same period one year before. Overall, FDA conducted 41 inspections in the seven months through May 2010, compared with 48 one year earlier.
Although this decline is more modest than that for the four-month period, it is still noteworthy, given GAO’s finding that the agency should be inspecting more foreign drug plants than it had been doing. Moreover, FDA appears to be losing ground in India in 2010 compared with earlier years as well.
For example, in fiscal 2007, according to GAO, FDA conducted 64 drug plant inspections in India. In fiscal 2010, the agency has completed 41 inspections through May, which puts it on track to complete 70 audits for the year. However, Saxon says FDA traditionally does not schedule inspections in India during the summer months, when heavy monsoon rains make business travel to India impractical. If inspections halt over the summer, then the agency will complete fewer than 70, and possibly fewer than 64.
That the number of inspections appears to be dropping raises the question of whether the agency is making progress in improving its diligence abroad. “It’s really not logical that the number of inspections would decrease,” Saxon says.
On the basis of his review of FDA’s inspection logs, Saxon finds another potential problem with the agency’s work in India. The inspection logs for the two seven-month periods shows that the vast majority of visits are for first-time approval of manufacturing facilities or for inspections linked to the launch of a new product at a facility that FDA has already inspected. The agency has been conducting almost no reinspections of facilities that have already been approved.
According to GAO’s 2008 report, reinspections of approved facilities traditionally account for 13% of all inspections. The FDA logs for November 2009 through May 2010 and the same period one year before list only four audits as reinspections of already approved facilities, or the equivalent of 4% of all inspections conducted during that time.
This suggests that inspectors have been concentrating on inspecting plants that are about to launch new products into the U.S. and delaying visits to facilities that have already been approved and that are not suspected of having developed manufacturing shortcomings.
The logs of inspections conducted in India this year and late last year detail a noticeable drop in the regularity of audits. But more broadly and seriously, they illustrate FDA’s lack of progress in monitoring the country that supplies more generic drugs in the U.S. than any other.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter