Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Mylotarg Must Go
Regulatory Action: The only antibody-drug conjugate on the market has efficacy and safety issues
by Ann M. Thayer
June 28, 2010
| A version of this story appeared in
Volume 88, Issue 26
Pfizer has agreed to stop selling the cancer drug Mylotarg, an antibody-drug conjugate (ADC) that has been on the market since 2000. FDA made the request after recent clinical studies failed to show any benefit from the drug and actually found that more patients taking it died than those who were receiving chemotherapy alone. Fewer than 2,500 patients in the U.S. receive Mylotarg annually, Pfizer says.
Because Mylotarg was thought to address an unmet medical need, it was approved on the basis of limited data under an accelerated process—but only to treat patients who are age 60 and older with relapsed acute myeloid leukemia. Under the process, Wyeth, which Pfizer acquired last year, was required to conduct postapproval studies to show the drug’s benefit.
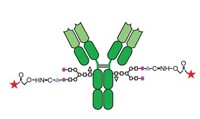
Mylotarg is the first, and to date the only, ADC to reach the market. It consists of a monoclonal antibody—targeting CD33-positive cancer cells—coupled by a chemically labile linker to a highly potent cytotoxic agent. Intended to be potent yet selective, ADCs are long-sought-after “magic bullets” in drug development, says Enrico T. Polastro, a Brussels-based senior industry specialist at the consulting firm Arthur D. Little.
Mylotarg, however, has had limited commercial success. Beyond the restricted patient population, another drawback is its small therapeutic window, or the narrow range between an effective and an adverse dose. The drug is linked to potentially fatal liver toxicity; the occurrence of this condition has increased with the drug’s use.
The drug’s weakness is likely due to the chemical linker, which relies on a pH-dependent release mechanism and might not be sufficiently stable, according to a recent review article by researchers at the Swiss life sciences firm Lonza (Bioconjugate Chem. 2010, 21, 5). If this is the case, too much cytotoxin can be released in the bloodstream instead of being delivered to the target.
To overcome such problems and advance new ADC candidates through clinical trials, several drug companies are trying to develop improved linkers (C&EN, Dec. 14, 2009, page 26). Although researchers continue to design and synthesize ADCs, they have yet to prove that they can overcome the practical difficulties, Polastro says. Seeing the only approved product withdrawn “won’t reassure people,” he adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter