Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Amyloid’s Functions Expand
Molecule that is linked to alzheimer’s might do more than just cause disease
by David Pittman
July 5, 2010
| A version of this story appeared in
Volume 88, Issue 27

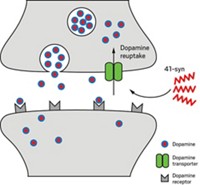
“Amyloid-β” has become a four-letter word for some researchers of Alzheimer’s disease.
The pesky peptide has long been seen as a possible cause of Alzheimer’s. It clumps into aggregates that could trigger the disease by mucking up brain tissue and interfering with normal neurological processes.
Evidence is mounting, however, that amyloid-β plays non-disease-causing roles in the brain. “Have we established that to be so?” asks Robert D. Moir, an Alzheimer’s researcher at Massachusetts General Hospital and Harvard Medical School. “No, not yet,” he says, “but we found evidence to suggest that it may be true.” This view is also supported by the existence of molecules capable of forming aggregates similar to those involved in Alzheimer’s, but that are beneficial to organisms.
Some researchers now think amyloid-β could be a foot soldier in the brain’s immune system and possibly a stimulant for proper brain function. In addition, Swiss scientists have gathered evidence that human hormones are stored in an amyloid-like conformation. Some bacteria use the amyloid structures’ cementlike qualities for their benefit.
In the human brain, enzymes chop up a membrane protein known as amyloid precursor protein to produce amyloid-β. These molecules readily stick together into oligomers rich in cross-β-sheet structures. Some oligomers are swept out of the cell, but others grow to form insoluble fibers that become the telltale plaques found in Alzheimer’s patients.
Most efforts in Alzheimer’s drug development focus on either clearing the brain of already-formed amyloid or preventing its formation altogether. With the latter approach, most companies are searching for small molecules that block γ-secretase, the enzyme that cuts the precursor protein to produce amyloid-β (C&EN, April 5, page 12).
But evidence of amyloid-β’s positive role is emerging. For example, Moir and coworkers at Harvard suggest that amyloid might be a “prison” that the brain uses to trap and immobilize microbial invaders, holding them in place while other parts of the brain’s innate, or nonspecific, immune system come to kill them (PLoS One 2010, 5, e9505).
“Like any immune response, it can get out of hand and give you a disease,” Moir says. “My data suggest that amyloid is not necessarily a response to a specific bug, unlike many diseases where you have one species of pathogen that gives you a specific disease. This one would be a generalized response to any type of infection or challenge in the brain.”
Brian J. Balin of Philadelphia College of Osteopathic Medicine supports the idea that amyloid-β could be part of an infection-control mechanism. His research suggests that the bacterium Chlamydophila pneumoniae triggers late-onset Alzheimer’s.
Balin found DNA of C. pneumoniae in 17 of 19 postmortem brain samples from Alzheimer’s patients, compared with only 5% of samples from similar-aged people without the disease (J. Alzheimers Dis. 2008, 13, 371).
“Furthermore, we have seen in vitro that amyloid can block infection of cells if it is present in the media prior to trying to infect the cells” with C. pneumoniae, Balin says. “We also believe that amyloid accumulation can be toxic, so the double-edged sword is what we may be left with.”
Other work suggests amyloid-β might benefit brain activity in an entirely different way.
Joost Schymkowitz of the Free University of Brussels found that an amyloid-β chain that is 40 amino acids long stimulates neuron activity in cultures grown in vitro. His research group measured the electronic activity of neuron synapses with a multielectrode array.
The addition of amyloid-β42, the specific 42-amino-acid form of the peptide associated with Alzheimer’s, reduced neuron activity. But amyloid-β40 gave a small stimulatory effect, which “suggests it actually has a function,” Schymkowitz says. He adds that amyloid-β production increases after brain injury, leading him to believe that amyloid stimulates proper brain function.
The two peptides differ in the location at which an enzyme cleaves the amyloid precursor protein during formation, leaving one longer or shorter. Schymkowitz eventually hopes to understand what triggers the formation of the toxic 42-amino-acid-long amyloid. And he questions whether it’s a good idea to eliminate the peptide’s production, which is the path most drug development is taking.
Amyloid-β’s function in the human brain is still being clarified, but amyloid structures are at work elsewhere in the body and in lower organisms.
Beyond the brain, the amyloid protein Pmel17 promotes the formation of melanin in human skin cells. This brownish black pigment determines skin color.
In addition, Roland Riek of the Swiss Federal Institute of Technology, Zurich, and coworkers found that hormones produced by the human endocrine system adopt amyloid-like β-sheet conformations when stored in granules in cells (C&EN, June 22, 2009, page 34). The fibrils can disassemble to release hormone molecules when needed.
Certain forms of amyloid biomaterials, which strongly resist degradation, have been found in the protective membranes of insect and fish eggs. And Daniel E. Barlow of the Naval Research Laboratory, in Washington, D.C., recently found that β-sheets of amyloid proteins are a key component of the hardy cement that gives barnacles their sticking strength (C&EN, March 1, page 34).
Amyloid fibers normally hold microbial communities together, says Richard M. Losick, a professor of molecular and cellular biology at Harvard University. Losick and his group recently found that amino acids in the rare d-form, rather than the normal l-stereochemistry, break up those amyloid-cemented biofilms (C&EN, May 3, page 8).
“Certainly, proteins in the amyloid state are thought to be associated with disease,” Losick says. “But more recently, examples of amyloid-like proteins in bacteria and fungi have cropped up in which the amyloid state is used for something biologically beneficial.”
The concept that amyloid can be functional is relatively new, Harvard’s Moir says. For decades, the peptide was viewed purely as a pathogenic agent. The overwhelming majority of work looking at its other functions and possible benefits has been done on lower organisms. Research on people, which is more difficult, is just starting, he notes.
“Has amyloid been demonstrated in higher organisms like us to do anything yet?” Moir asks. “Not really. But there’s a certain growing sense that it might be the case.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter