Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
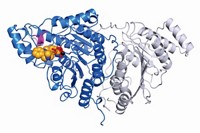
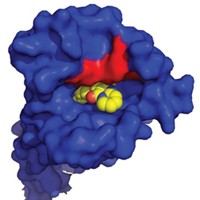
Flexible Drug Target Reveals Its Secrets
Crystal structure helps elucidate the conformational flexibility of enzymes, offering new insights for designing drugs
by Sarah Everts
January 18, 2010
| A version of this story appeared in
Volume 88, Issue 3

The first X-ray crystal structure of a flexible member of an enzyme family involved in diseases ranging from cancer and diabetes to inflammation and asthma could bolster efforts to develop drugs that selectively inhibit the enzymes. Pharmaceutical companies trying to develop drugs aimed at the phosphoinositide-3-OH kinases (PI3Ks) have been stymied by an inability to target specific family members and therefore minimize side effects from interactions with other members. A team led by Roger L. Williams of the Medical Research Council’s Laboratory of Molecular Biology, in Cambridge, England, solved the structure of the δ isoform of PI3K bound to several inhibitors that were flat or shaped like propellers (Nat. Chem. Biol., DOI: 10.1038/nchembio.293). This isoform, associated with inflammation, has a topology similar to other structurally characterized PI3K isoforms, but it appears to be more flexible. For example, the team found that propeller-shaped inhibitors squeeze into a site normally occupied by the energy-giving molecule ATP. In order to fit, the inhibitors create a new cavity in a section of the enzyme that is not normally open. Learning more about the conformational flexibility of enzymes that otherwise look the same could be key to designing drugs specific to a particular isoform, Williams says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter